RESEARCH AND DEVELOPMENT OF NEW DRUG PRODUCTS
Introduction. The study of 1,3-oxazine derivatives plays a crucial role in the field of heterocyclic chemistry. However, there is a lack of systematized information in the literature regarding the synthesis methods of bis-derivatives of 1,3-oxazin-6-ones. The development of efficient methods for obtaining these oxazines, as well as the study of their structure, properties, and biological activity, represents a promising direction for both medicinal chemistry and the pharmaceutical industry.
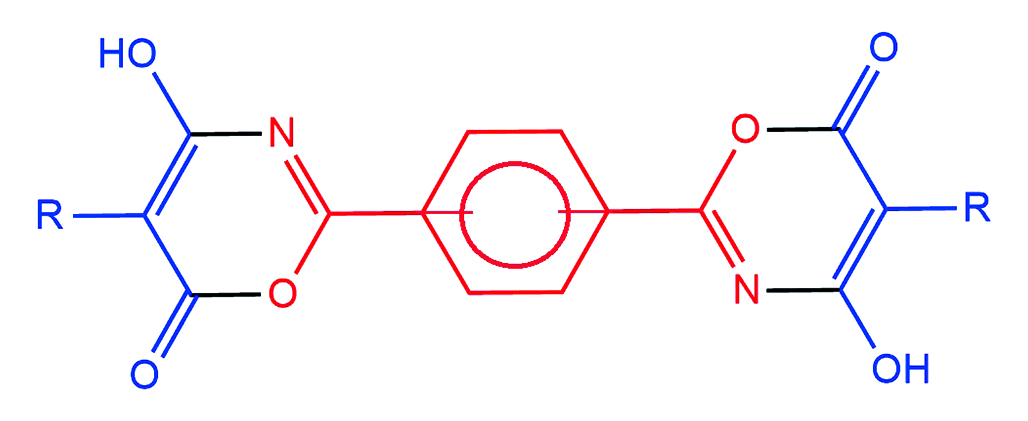
Aim. To develop a laboratory method for the synthesis of bis(4-hydroxy-6H-1,3-oxazin-6-ones) based on the reaction of benzene-1,3-dicarboxamide and benzene-1,4-dicarboxamide with substituted malonyl chlorides, and to confirm their structure using nuclear magnetic resonance (NMR) spectroscopy on 1H and 13C nuclei.
Material and methods. 1H and 13C NMR spectra were recorded on a Bruker AM-600 spectrometer in deuterated dimethyl sulfoxide (DMSO-d6), and their processing was carried out using ACD/Labs software. The progress of the reactions was monitored using thin-layer chromatography on plates coated with silica gel TLC Silicagel 60 F254 using ethyl acetate as the eluent and detection in ultraviolet (UV) light. The synthesis procedure included suspending 1 mmol of terephthalic or isophthalic acid diamide in absolute 1,2-dichloroethane, adding 2,4 mmol of substituted malonyl chloride and refluxing the mixture for 15 hours. The end of the reaction was determined by the absence of the initial diamides in the reaction mass by thin-layer chromatography.
Results and discussion. As a result of the study a laboratory method of the synthesis of bis(4-hydroxy-6H-1,3-oxazine-6-ones) with phenylene bridges between heterocyclic fragments was developed. The use of absolute 1,2-dichloroethane as a solvent made it possible to increase the yield of the target compounds by 5–7 % and reduce the synthesis time by 5 hours compared to absolute benzene. The structure of the obtained compounds was proved by nuclear magnetic resonance spectroscopy on 1H and 13C nuclei. The spectra of the obtained compounds contain all the characteristic signals corresponding to bis(4-hydroxy-6H-1,3-oxazine-6-ones).
Conclusion. A laboratory method for the synthesis of bis(4-hydroxy-6H-1,3-oxazin-6-ones) has been developed, implemented, and optimized based on the reaction of benzene-1,3-dicarboxamide and benzene-1,4-dicarboxamide with monosubstituted malonyl chlorides. The target compounds were obtained in absolute 1,2-dichloroethane with yields close to quantitative, confirming the efficiency of the developed approach. The structure of all obtained bis(4-hydroxy-6H-1,3-oxazin-6-ones) was reliably established using nuclear magnetic resonance spectroscopy on 1H and 13C nuclei.
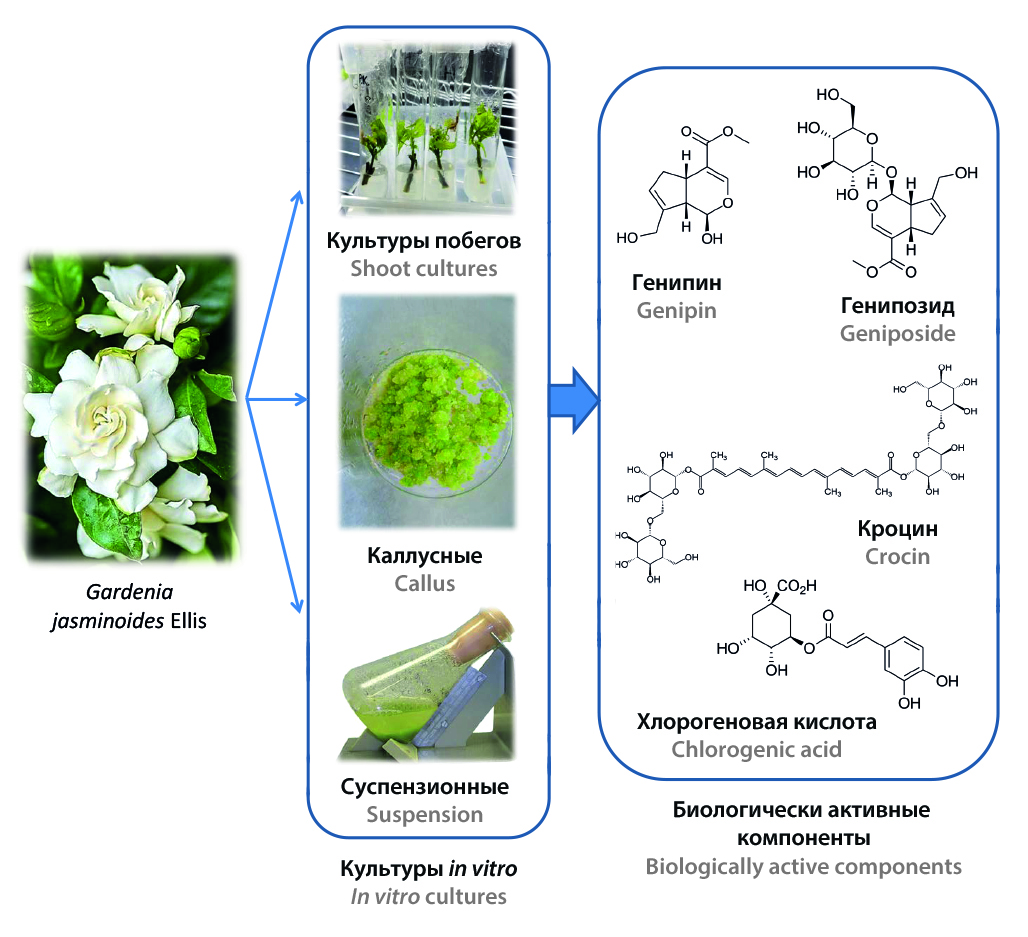
Introduction. Gardenia jasminoides Ellis is an evergreen shrub of the Rubiaceae family, naturally growing in China and Japan, and cultivated as an ornamental plant in many regions of the world. This review presents data on the results of studies of the accumulation of biologically active substances in various in vitro cultures, the possibility of inducing their biosynthesis, and the biological activity of substances isolated from Gardenia jasminoides cell cultures.
Text. To date, about 162 compounds have been isolated and identified from G. jasminoides, including flavonoids, iridoid glycosides, gardenia yellow pigment, monoterpenoids, sesquiterpenoids, triterpenoids, organic acids and their derivatives. The most important biologically active constituents of G. jasminoides are iridoid glycosides and yellow pigments – crocin derivatives. The use of plant cell cultures is one of the most promising approaches to obtaining biologically active substances of plant origin, since this method is characterized by less dependence on climatic and environmental factors, provides more precise process control and allows for a shorter production cycle, which contributes to the effective scaling of production. A number of researchers have obtained callus and suspension cultures, as well as shoot and modified root cultures of G. jasminoides. In order to increase the production of the main classes of biologically active substances – iridoid glycosides, polyphenolic compounds and carotenoids in callus, suspension and shoot cultures of G. jasminoides, a number of studies were conducted on the introduction of specific additives into nutrient media. It was shown that G. jasminoides cell cultures have high antioxidant activity due to phenolic compounds such as ferulic and chlorogenic acids. Callus culture extracts showed significantly greater superoxide dismutase activity than leaf extracts. At the same time, only callus culture extracts exhibited antimicrobial activity against Escherichia coli and Bacillus cereus.
Conclusion. A review of the literature data allows us to conclude that in vitro G. jasminoides cultures provide stable and enhanced production of valuable secondary metabolites (iridoid glycosides, polyphenols, carotenoids), exceeding the indicators of intact plants, which opens up prospects for the industrial production of biologically active substances and phytopreparations.
ANALYTICAL METHODS
Introduction. Overexpression of the HER2 receptor is associated with aggressive cancer progression and poor prognosis. Traditional immunohistochemical diagnostics have a number of limitations, including invasiveness, inability to assess tumor heterogeneity, and total spread of the process. A promising alternative is radionuclide imaging using targeted scaffold DARPin G3 proteins. Based on the results of preclinical studies, the drug 99mTc[Tc]-G3-(G3S)3C has been proposed for Phase I clinical trials. The experimental drug is a sterile lyophilizate of a chemical precursor in a single vial and must comply with the requirements of OFS 1.11.0005 "Chemical Precursors for Radiopharmaceuticals" and OFS 1.7.1.0007.15 "Medicinal Products Obtained by Recombinant DNA Methods."
Aim. The aim of the present study was to develop approaches and techniques for quality control of DARPin G3-(G3S)3C protein included in the developed composition of chemical precursor lyophilizate lyophilizate for preparation of 99mTc-containing preparation for radionuclide imaging of HER2 overexpression in malignant tumors
Materials and methods. A lyophilizate containing DARPin G3-(G3S)3C with excipients was prepared for quality control. HPLC with a tandem mass spectrometer equipped with an electrospray ionization source was used to record mass spectra. Vertical electrophoresis with SDS in a heterogeneous buffer system was carried out in a polyacrylamide gel with an acrylamide concentration of 15 % at 110 V for 2 hours. Coomassie B-250 solution was used as the dye solution. Peptide mapping was performed using an HPLC system with a mass spectrometer via a nano-electrospray source. When correlating the amino acid sequence, carbamidomethylation of Cys, deamidation of Asn/Gln and oxidation of Met were counted as variable modifications. UV spectrophotometry was performed to determine the Quantification index at a wavelength of 280 nm. Validation evaluation of the developed method was carried out in accordance with the requirements of OFS.1.1.0012 "Validation of analytical methods".
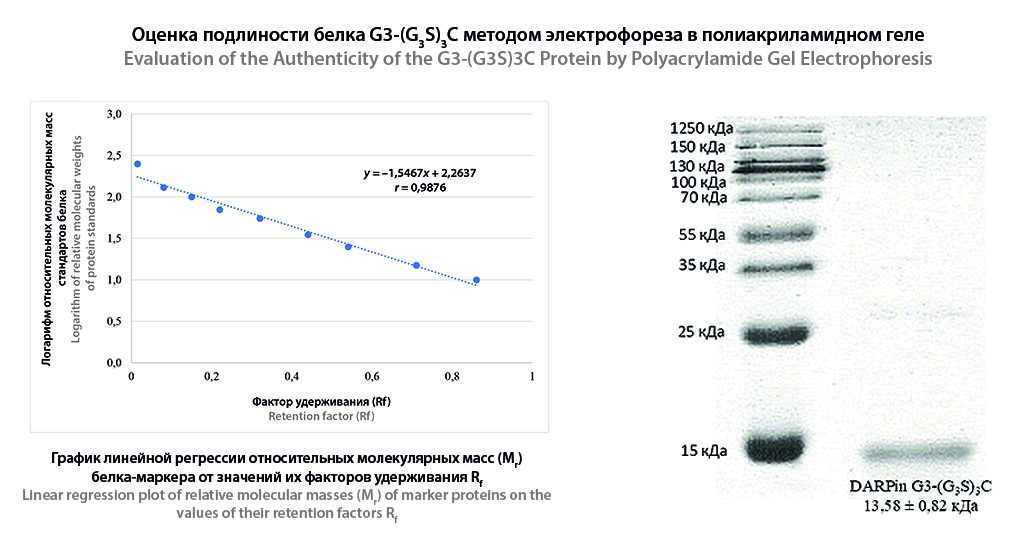
Results and discussion. The authenticity and purity of DARPin G3-(G3S)3C was confirmed using HPLC-MS. The purity of the protein was close to 100 %. Application of 2 μg of protein for electrophoresis in polyacrylamide gel showed a distinct stain allowing the authenticity of DARPin G3-(G3S)3C to be established. To determine the homodimer impurity, 10 μg of protein should be applied. The purity of the protein established by the peptide mapping method is 98 %. More than 700 variations of peptide fragments were identified by protein hydrolysate analysis. The value of –10LogP was more than 20. The developed UV-visible spectrophotometry technique meets the validation requirements and allows the quantification of DARPin G3-(G3S)3C protein in the lyophilizer ±10 % of the nominal content.
Conclusion. To determine the "Genuineness" of a new chemical precursor of DARPin G3-(G3S)3C protein in the lyophilizate for the preparation of 99mTc-containing preparation for radionuclide imaging of HER2 overexpression in malignant tumors the following methods were proposed: mass spectrometry, peptide mapping, electrophoresis in polyacrylamide gel. For the indicator "Related impurities" – the technique of electrophoresis in polyacrylamide gel. For the indicator "Quantitative determination" the method of UV-spectrophotometry.
Introduction. The study of the qualitative and quantitative composition of plants and the identification of promising medicinal species is one of the most important tasks of modern pharmacognosy. Yellow loosestrife (Lysimachia vulgaris L.) of the Primulaceae family is a perennial herbaceous plant. The main group of active substances in the herb of L. vulgaris are phenolic compounds. L. vulgaris is widely distributed in Russia, is known in traditional medicine, its biological activities have been examined in vitro and in vivo experiments. A search of scientific literature in the Russian segment did not reveal sufficient information on the quantitative content of biologically active substances.
Aim. Development and validation of an analytical procedure for assessing the quantitative content of flavonoids in the herb of yellow loosestrife (L. vulgaris) in terms of rutin using spectrophotometry.
Materials and methods. The object of the study was alcohol extracts from the aerial part of L. vulgaris, harvested during the flowering period in the Leningrad region. Quantitative determination was carried out by spectrophotometry. The proposed analytical procedure was validated in accordance with the requirements of the State Pharmacopoeia XV in terms of specificity, linearity, range, repeatability (intra-assay precision), precision and accuracy (trueness).
Results and discussion. For the proposed analytical procedure, rutin was proposed as a standard sample. It was found that the maximum extraction of flavonoids from the herb of L. vulgaris is achieved by using a single extraction with 70 % ethyl alcohol for 30 min at a raw material : extractant ratio of 1 : 100 and a raw material grinding of 2–1 mm. A 1 % alcohol solution of aluminum chloride was proposed as a complexing agent, and the complexation conditions were selected (aliquot of aluminum chloride 1 % solution 2 ml, complexation time 30 min). The analytical procedure meets the requirements of the validation characteristics. According to the developed method, it was found that the amount of flavonoids in the herb of L. vulgaris, harvested in the Leningrad region, is 2,97 ± 0,05 % expressed in rutin.
Conclusion. A method for the quantitative determination of the flavonoid content in the herb of yellow loosestrife (L. vulgaris) by spectrophotometry expressed in rutin has been developed and validated.
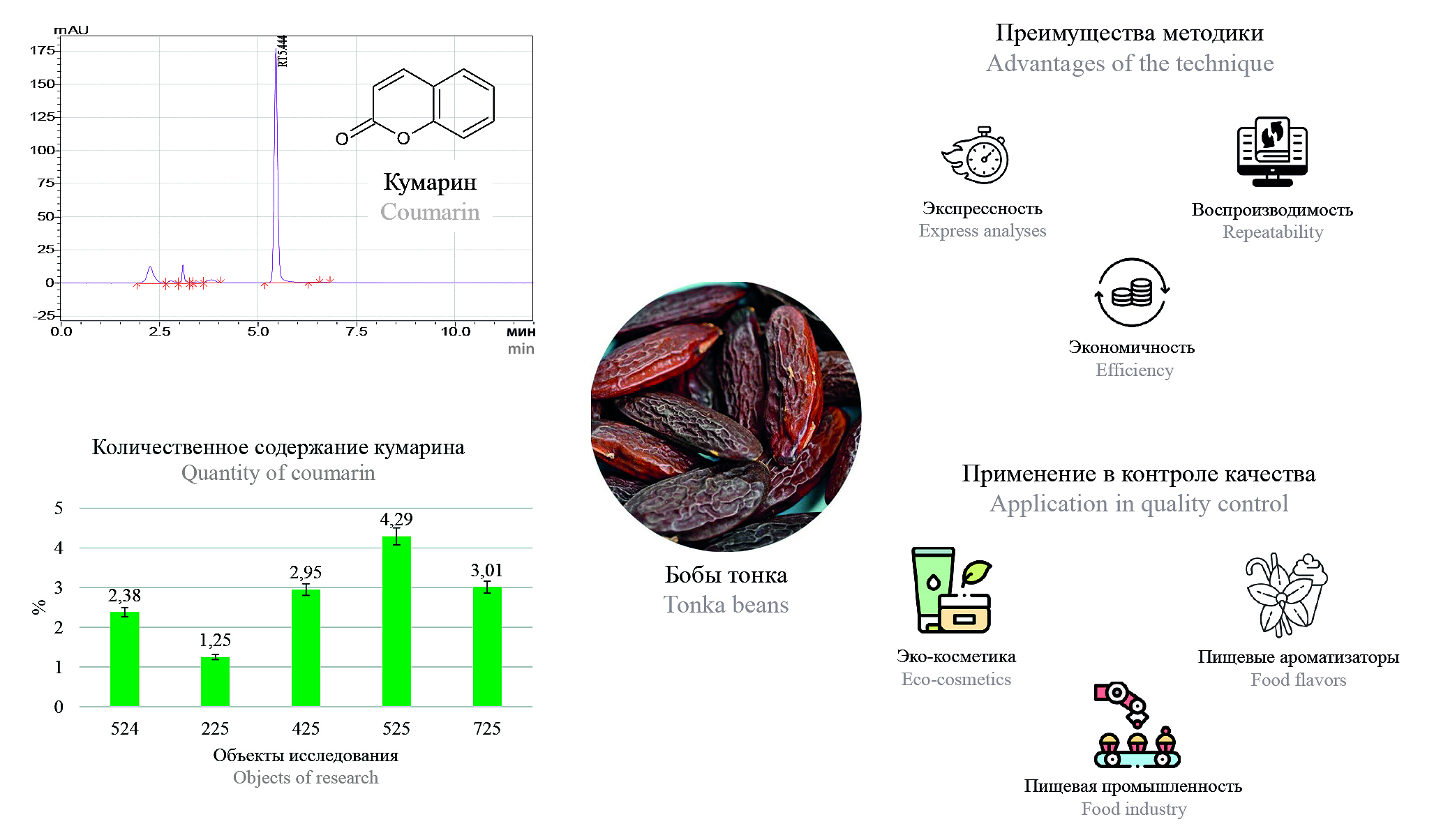
Introduction. The tonka beans (seeds of Dipteryx odorata, family Fabaceae) is a valuable source of coumarin used in the food, perfume and cosmetic industries thanks to its rich aroma. Despite its pharmacological value (anticoagulant, antioxidant activity), coumarin has a proven hepatotoxicity and haematotoxicity, which limits its use in a number of countries. Despite the control of synthetic coumarin in aromatics, the natural analog of tunica beans does not have clear rules, although it has identical activity. Growing demand for organic products has increased the popularity of tonka beans, but lack of scientific data on their safety and methods of analysis makes quality control difficult. The development of validated techniques, such as HVACR, is an urgent task to ensure the safety of its application in industry.
Aim. Development of a HPLC-method for the quantitative determination of coumarin in beans and its validation with further testing on several batches of the studied object.
Materials and methods. Five batches of Tonka beans from different producing countries were purchased as research objects, for sample preparation was used US-bath. Chromatography conditions: Luna 5 μm C18(100) Å LC Column 250 × 4.6 mm, thermostat temperature – 40 °C, detection wavelength – 275 nm. Mobile phase A – acetonitrile, mobile phase B – water. Elution mode: isocratic mobile phase B – 50 % for 12 minutes.
Results and discussion. The method meets the criteria of suitability of the chromatographic system, specificity, linearity (range 0.001–0.1 mg/ml) and precision. Its advantages are expressiveness and economy, which simplifies the quality control of raw materials. The quantitative content of coumarin in the studied object ranges from 1.25 ± 0.07 % to 4.29 ± 0.12 %.
Conclusion. Developed and validated by HPLC-methodology for the quantitative determination of coumarin in tonka beans. The developed methodology can be used to standardize this promising raw material in various industries, ensuring the necessary level of safety in its application.
PRECLINICAL AND CLINICAL STUDIES
Introduction. Physiologically-based pharmacokinetic modelling is a method that allows predicting the distribution of drugs in the body based on anatomical and physiological parameters. This approach has become widespread only with the development of computing technologies. Today PBPK is actively used by regulatory agencies to optimize clinical trials and reduce the number of animal experiments.
Text. PBPK models represent the body as a system of interconnected compartments corresponding to organs and tissues. Three main types of models are described: Full PBPK, which maximizes accuracy at the expense of detail; Reduced PBPK, which reduces computational complexity; Hybrid PBPK, which combines both approaches to balance accuracy and efficiency. Key parameters for model building are discussed in detail: physicochemical properties of substances (LogP, pKa, solubility), physiological parameters (organ volumes, blood flow, enzyme activity and membrane transport proteins) and pharmacokinetic parameters (volume of distribution, clearance). Special attention is given to the Gordon Amidon absorption and transit model (CAT/ACAT) and its integration into PBPK modeling. Procedures for model reliability are given calibration (parameter tuning), validation (assessment of predictive ability), qualification (confirmation of fitness for purpose), and verification (verification of mathematical correctness). Statistical metrics for assessing accuracy are described. An overview of popular PBPK modeling software such as GastroPlus, Simcyp, PK-Sim, SimBiology, and Mrgsolve is presented, highlighting their main advantages and applications in the pharmaceutical industry and academic research.
Conclusion. PBPK modeling is on the threshold of a new era where its application will go beyond traditional pharmacokinetics, becoming an integral part of digital medicine, biotechnology and precision therapeutics. In the future, such technologies will not only be able to predict the behavior of drugs in the body, but also become the basis for virtual clinical trials, which will fundamentally change the approach to drug development and application.
Introduction. Hedysarum semenowii Regel & Herder of the genus Hedysarum L., possess a rich phyto-chemical profile and hold promise as a foundation for developing novel pharmaceuticals. Despite the recognized pharmacological activities of various Hedysarum L. species, their safety profiles have not been sufficiently studied, necessitating further investigation to evaluate their toxicity and potential adverse effects. To address this gap, a study was conducted to assess the subacute toxicity, local irritant effects, and allergenicity of Hedysarum semenowii extract, with the aim of determining its safety and therapeutic potential.
Aim. Determination of subacute toxicity, local irritant effect and allergenicity of the Hedysarum semenowii Regel & Herder extract.
Materials and methods. The subacute toxicity study was performed using outbred rats, with the extract from aerial part of the plant administered orally at doses of 500, 2000, and 5000 mg/kg over a 28-day period. The condition of the animals was monitored throughout the study. At the conclusion of the subacute toxicity evaluation, pathological changes in the liver and kidneys of the experimental animals were examined. Local irritant and allergenic effects were assessed in rats and guinea pigs, respectively, employing visual inspection and the Draize scoring system.
Results and discussion. The 28-day subacute toxicity study revealed no significant toxicity, mortality, or behavioral changes in the animals. Body weight, food consumption, and water intake remained stable across all groups. According to the results of macroscopic and microscopic examination, minor physiological changes were noted in the liver and kidneys, the severity of which correlated with the administered doses of the extract. Furthermore, the extract did not induce skin irritation or allergenic reactions in tests involving rats and guinea pigs.
Conclusion. Hedysarum semenowii extract exhibited low toxicity and an absence of irritant and allergenic effects, affirming its safety and potential for further exploration as a therapeutic agent.
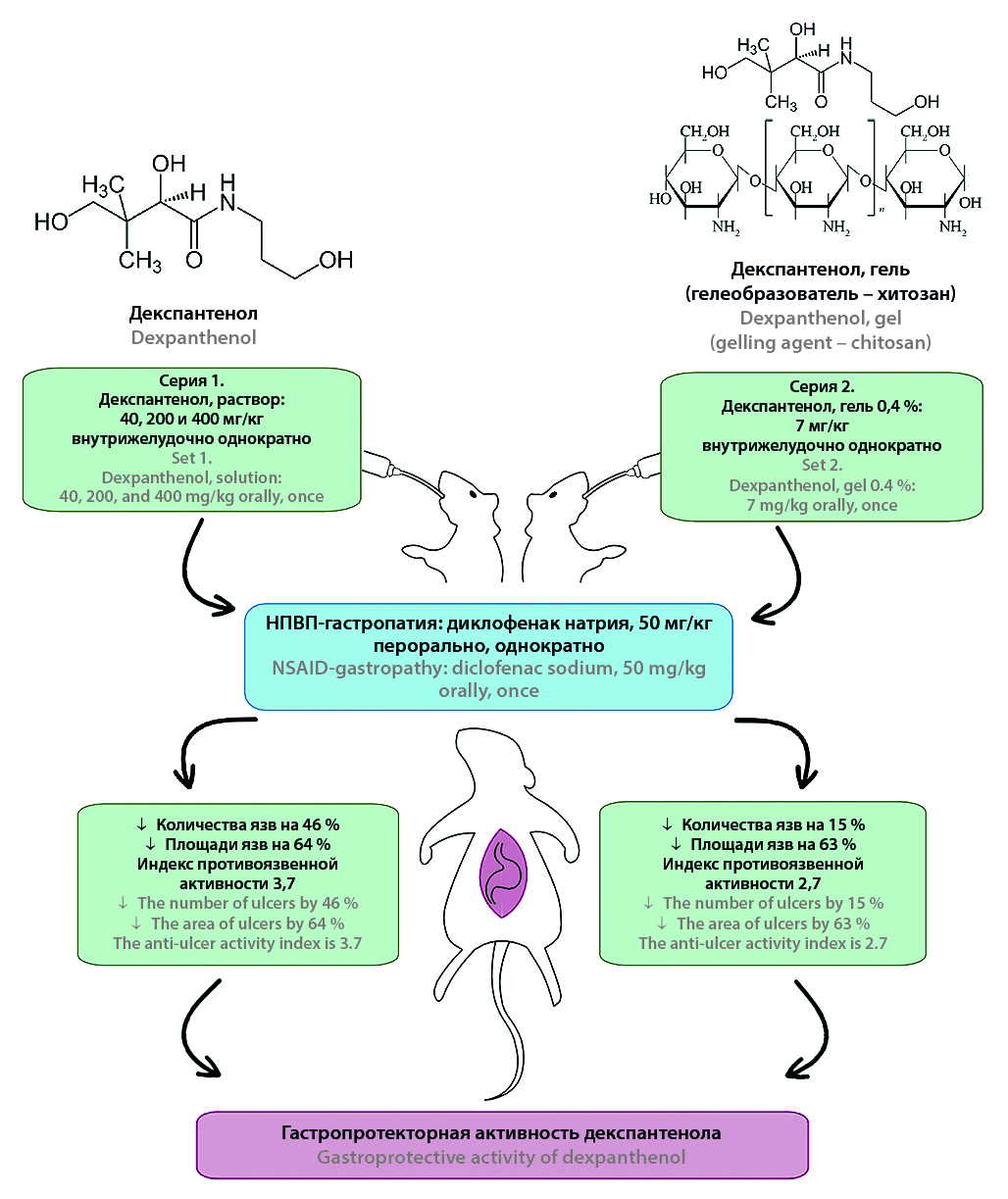
Introduction. The search for new gastroprotectors remains relevant, one of which could be dexpanthenol – a precursor of pantothenic acid that is non-toxic when taken orally. In Russia, systemic dexpanthenol preparations are not registered, and their use for treating acid-related diseases (ARDs) of the gastrointestinal tract isn’t known, and studying its gastroprotective properties when administered orally is promising.
Aim. Study of gastroprotective properties of dexpanthenol in oral administration using a model of gastropathy caused by non-steroidal anti-inflammatory drugs (NSAID-induced gastropathy) in preclinical research.
Materials and methods. The studies were conducted on 142 male outbred white rats weighing 220–280 g. The model used was NSAID-induced gastropathy with ulcerogenic agent sodium diclofenac, administered orally in a single dose of 50 mg/kg, euthanasia was performed 3 hours after administration via an overdose of chloroform anesthesia. Dexpanthenol was administered 1 hour before sodium diclofenac in a single oral dose in the form of an aqueous solution at doses of 400, 200, 40 mg/kg, and in the form of a developed 0.4 % gel (using high-viscosity chitosan as a gelling agent) at a dose of 7 mg/kg. Comparative drugs: dioxomethyltetrahydropyrimidine (syn. methyluracil) at a dose of 42 mg/kg, bismuth tripotassium dicitrate at a dose of 17 mg/kg. Both drugs were administered orally 1 hour before sodium diclofenac in a single dose. Evaluation criteria: number of animals with ulcers, number and area of ulcers on the gastric mucosa (GM) – determined using developed software written in Python with a graphical user interface (GUI). Calculation of the Pauls index and antiulcer activity index using known formulas, conducted histological examinations of the gastric mucosa with hematoxylin-eosin staining.
Results and discussion. It has been established that dexpanthenol provides a dose-dependent statistically significant reduction in the number and area of ulcers on the gastric mucosa, maximally by 2.7 times (at a dose of 40 mg/kg, 1,0 % aqueous solution, single oral administration) compared to the control group, and antiulcer activity index is 3.7, which exceeds the effectiveness of methyluracil. The 0,4 % dexpanthenol gel at a dose of 7 mg/kg demonstrated a significant reduction in ulcer area by 63 %, with an antiulcer activity index of 2.7, surpassing the effectiveness of bismuth tripotassium dicitrate. This is confirmed by signs of reduced inflammation intensity and activation of regeneration processes according to histological studies. The observed effects are likely attributed not only to dexpanthenol’s intrinsic activity but also to synergistic interactions with chitosan and the gastroprotective mucoadhesive properties of the gel formulation. The revealed gastroprotective activity of dexpanthenol aligns with its known mechanisms of action, including preservation of phospholipid bilayer integrity, modulation of mucin and tight junction (TJ) protein synthesis, regulation of nuclear transcription and apoptosis factors, growth factors, cytokines, and inflammatory mediators, cell division and cell differentiation.
Conclusion. For the first time, it has been proven that dexpanthenol, when administered orally in a single dose on the NSAID-gastropathy model, exhibit gastroprotective activity. It provides a significant statistically significant reduction in the number and area of ulcers compared to the control group. The antiulcer activity index is 3.7 (at a dose of 40 mg/kg, 1.0 % aqueous solution) surpassing the efficacy of methyluracil, 2,7 – when administered as a 0,4 % gel at 7 mg/kg (with chitosan as the gelling agent), these results were confirmed by histological examination and demonstrated superior effectiveness compared to bismuth tripotassium dicitrate.
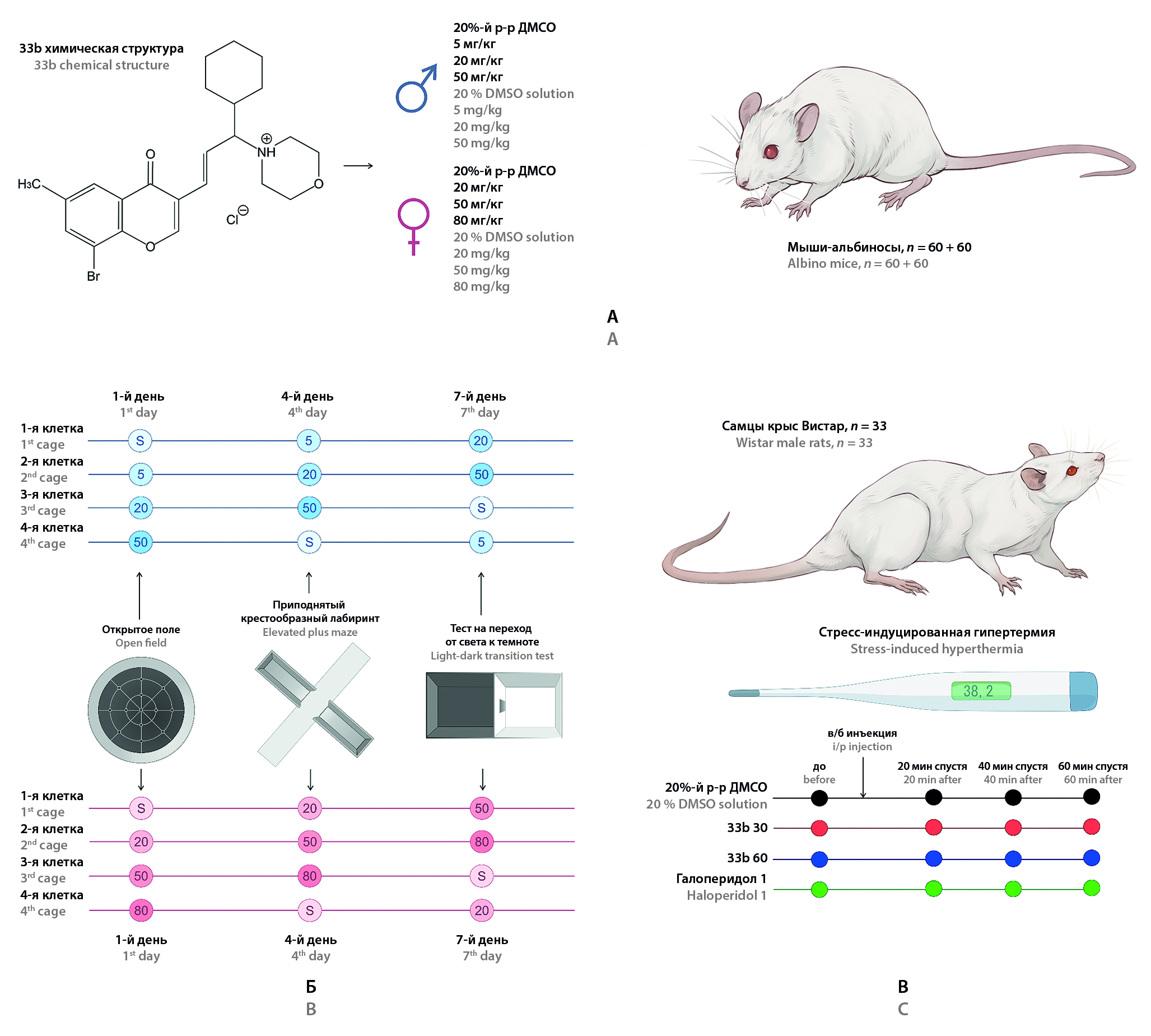
Introduction. Chromone-containing allylmorpholine derivatives (PAMs) are a new group of biologically active compounds with putative psychotropic activity. In a series of experiments on Danio rerio, PAMs demonstrated a pronounced dose-dependent sedative effect, and compound 33a at low doses exerted anxiolytic effects in the Novel tank test. Screening by pharmacoencephalography of another molecule from the PAM series, 33b, demonstrated similarity of its effects on EEG with the dopamine blocker sulpiride and the antihistamine agent chloropyramine. Based on the results of this prediction, the molecule can be hypothesized to have sedative and antipsychotic effects.
Aim. To study the pharmacological activity of compound 33b in tests for exploratory and anxiety behavior in mice, as well as in the test "Stress-induced hyperthermia" in rats.
Materials and methods. The pharmacological activity of 33b was evaluated in the Open Field (OF), Elevated Plus Maze (EPM), Light-Dark Box test (LDB) tests in male and female mice, as well as in the Stress-Induced Hyperthermia test in male Wistar rats.
Results and discussion. In the OF, EPM and LDB tests, the studied compound 33b at doses of 50 and 80 mg/kg resulted in a persistent decrease in the number of stance in both females and males. The decrease in locomotor activity in the OF test remained at trend level without statistical significance. Suppression of vertical activity in males occurred when lower doses of 33b were administered than in females. No data on the anxiolytic action of the molecule in these tests were obtained. In the test "Stress-induced hyperthermia" a pronounced decrease in body temperature was observed in rats when 33b was administered at a dose of 60 mg/kg.
Conclusion. The data obtained demonstrated a moderate sedative effect of compound 33b, while the sensitivity of males to the action of the molecule was higher. In the test on rats for the studied PAM was shown hypothermic action under stress conditions, which may be a confirmation of its dopamine-blocking action. This allows us to consider substance 33b from the group of chromone-containing allylmorpholines as a promising compound for further studies as a potential antipsychotic agent.
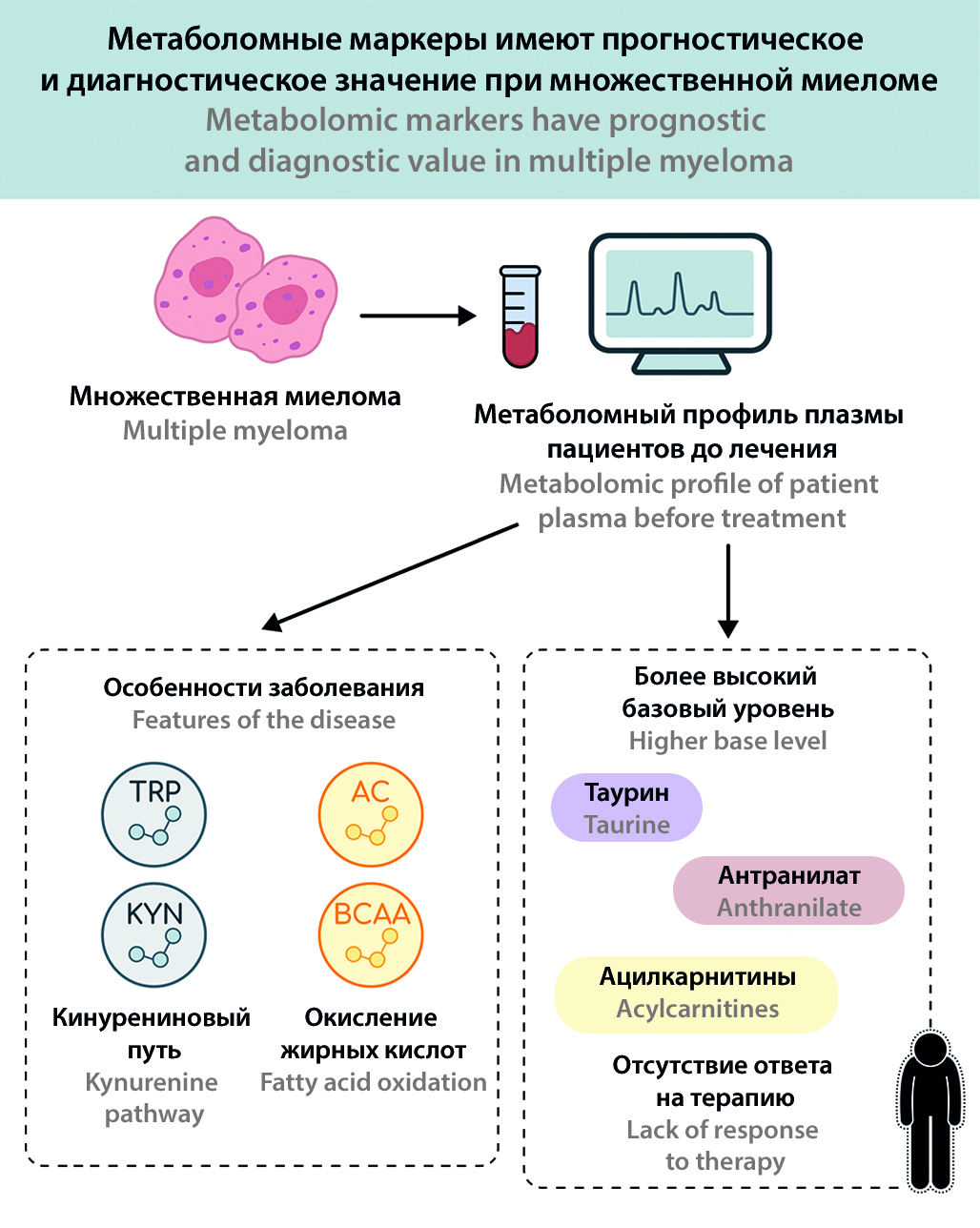
Introduction. Multiple myeloma (MM) is a malignant disease of plasma cells characterized by marked heterogeneity of the clinical course and variability in response to treatment. Metabolomic analysis, which reflects the totality of small molecules in biological fluids, opens up new possibilities for the search for diagnostic and prognostic biomarkers.
Aim. To evaluate metabolomic profiles of patients with multiple myeloma (MM) and to identify metabolic markers associated with the efficacy of polychemotherapy.
Materials and methods. The study was conducted from September 2022 to May 2025 at the Department of Hospital Therapy No. 1 of Sechenov University. We performed targeted analysis of plasma metabolites in 29 pre-treatment MM patients and 30 healthy volunteers (controls). Patients were divided into response and no response groups based on the results of therapy with VCD protocol after three courses.
Results and discussion. Significant differences in metabolomic profiles of MM patients compared to controls were found. MM patients showed increased tryptophan catabolism via the kynurenine pathway (~41 % increase in kynurenine/tryptophan ratio, ~80 % decrease in serotonin levels), changes in urea and nitric oxide cycle metabolites (~28 % decrease in arginine, ~5.3-fold increase in asymmetric dimethylarginine), and amino acid imbalances (decrease in serine, aspartate, BCAA) and a significant increase in total acylcarnitines (~1.4-fold higher than control). The baseline metabolic profile also differed between patients with different treatment outcomes: before treatment, patients who subsequently showed a clinical response had lower levels of several acylcarnitines and tryptophan breakdown products (e.g. anthranilic acid), whereas patients without response showed decreased levels of 5-hydroxytryptophan, indole-3-lactic acid and histidine.
Conclusions. Metabolomic analysis revealed characteristic metabolic alterations in MM reflecting activation of immunometabolic pathways (tryptophan kynurenine pathway, arginine metabolism) and impaired energy and amino acid regulation. The results indicate the potential prognostic significance of metabolites: a number of biomarkers (e.g. tryptophan derivatives, acylcarnitines) may be associated with chemotherapy sensitivity. The findings open the prospects for further research on metabolic approaches in MM monitoring and therapy.
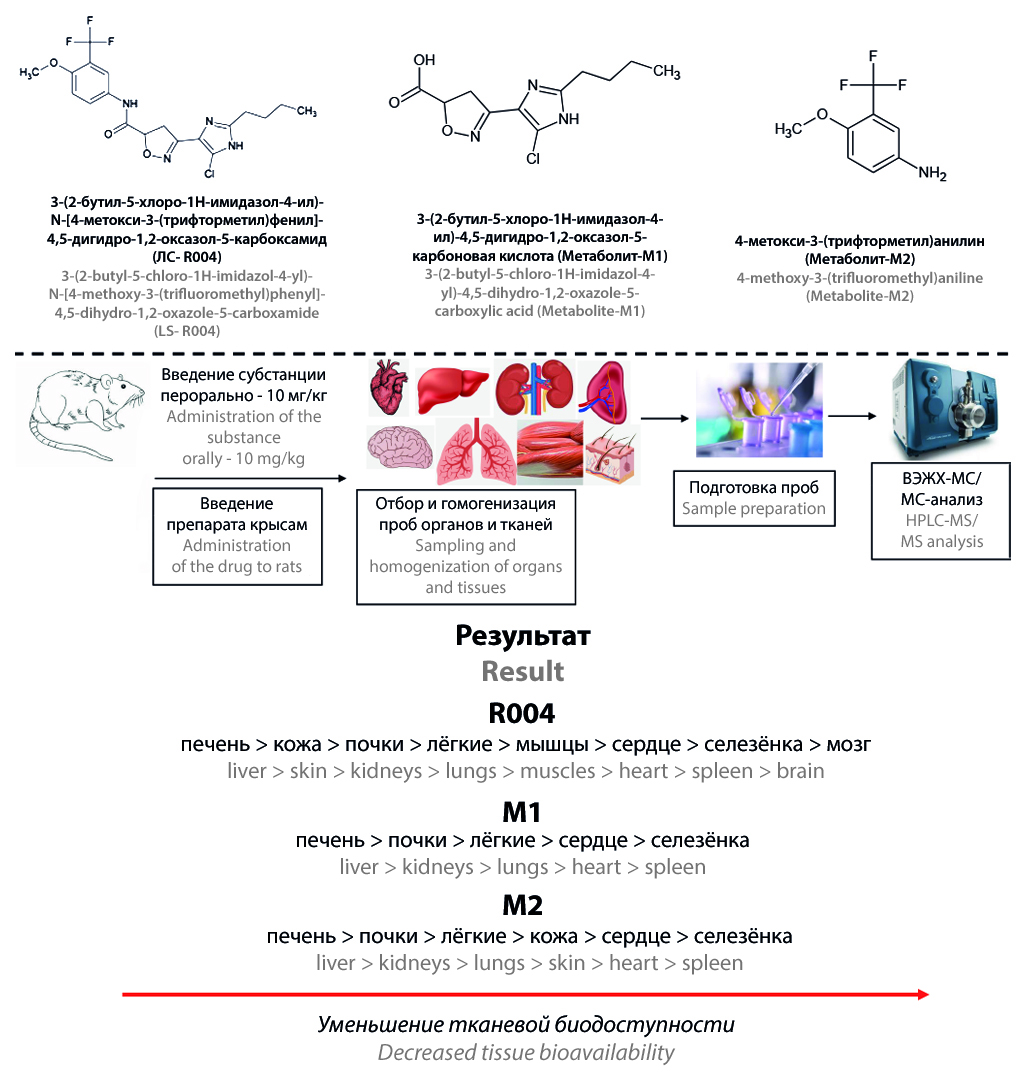
Introduction. The new pharmacologically active substance 3-(2-butyl-5-chloro-1H-imidazole-4-yl)-N-[4-methoxy-3-(trifluoromethyl)phenyl]-4,5-dihydro-1,2-oxazole-5-carboxamide (R004) is a new PAR-2 receptor inhibitor for treatment of rheumatoid arthritis. This compound is at the stage of preclinical trail. The distribution study of R004 and its metabolites by organs and tissues has not been performed before.
Aim. The investigation of distribution of R004 and its metabolites in rat organs after a single oral administration of the active substance.
Materials and methods. The study was carried out on 50 male Wistar rats. Substance of R004 was administered orally at a therapeutic dosage of 10 mg/kg. Sampling of liver, kidneys, heart, lungs, spleen, brain, skin and muscles was performed 1, 2, 3, 4, 6, 8, 10, 12, 16, 24 h after administration of the drug. Organs were immediately homogenized using acetonitrile and frozen. Further sample preparation was carried out by addition an acetonitrile solution of internal standards to the homogenates. Quantification of R004 and its metabolites 3-(2-butyl-5-chloro-1H-imidazole-4-yl)-4,5-dihydro-1,2-oxazole-5-carboxylic acid (M1) and 4-methoxy-3-(trifluoromethyl)aniline (M2) was performed by HPLC-MS/MS.
Results and discussion. The developed method for quantification of R004, M1 and M2 in organs has been successfully validated in the analytical ranges of 2–2000 ng/g for R004 and 1–1000 ng/g for M1 and M2. R004 is distributed by all studied biological objects. The tissue bioavailability of R004 decreases in the following sequence: liver > skin > kidneys > lungs > muscles > heart > spleen > brain. M1 and M2 was detected in large quantities in liver and kidneys. Metabolites penetrated into the heart, lungs and spleen in much lower concentrations. In skin tissues only M2 was identified. M1 and M2 were not found in brain and muscles.
Conclusions. The validated bioanalytical method has been successfully used for distribution study of R004 substance. The drug is well distributed throughout the studied organs and has a high tissue bioavailability. The highest concentrations of metabolites were observed in liver and kidneys.
PHARMACEUTICAL TECHNOLOGY
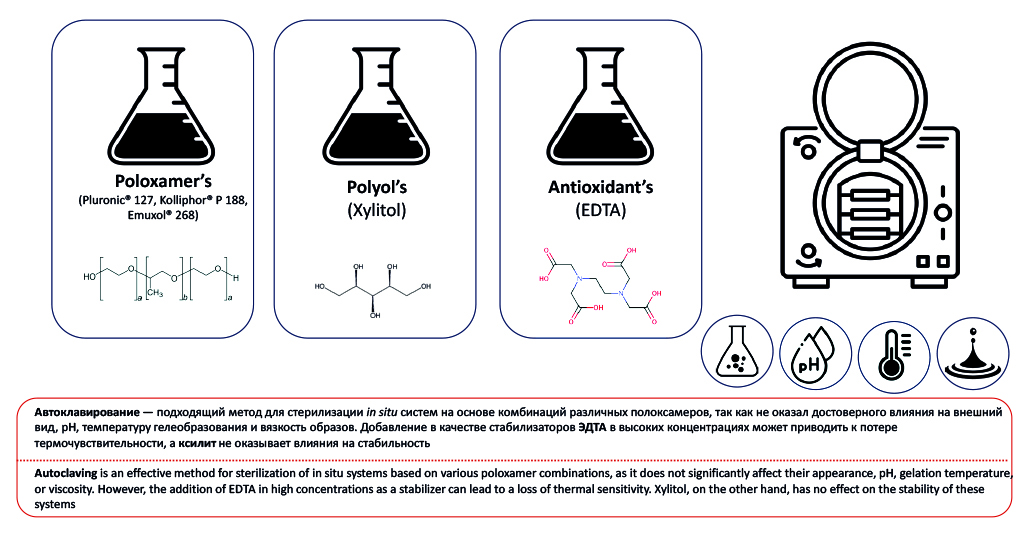
Introduction. Parenteral and ophthalmic in situ systems must be sterile. The selection of sterilization method is a key step in the development of sterile stimuli-sensitive systems, as an inappropriate method can lead to the degradation of the gel-forming polymer and the API, resulting in a loss of activity. Unfortunately, the stability of poloxamer-based thermosensitive systems during sterilization, as well as methods for their stabilization using protective agents, remains insufficiently studied.
Aim. The aim of the study was to investigate the stability of poloxamer-based systems during autoclaving and to develop methods for protecting them from the adverse effects of sterilization.
Materials and methods. Poloxamers used in the experiment included Kolliphor® P 407, Kolliphor® P 188, Kolliphor® P 338, and Kollisolv® P 124 (BASF, USA), as well as Emuxol-268 and Proxanol-168, provided by JSC "NIOPIK" (Russia). Disodium salt of ethylenediaminetetraacetic acid (EDTA) (LLC PCF "KhimAvangard", Russia) and xylitol ("Sladkiy Mir" LTD, Russia) were selected as protective agents. The samples were autoclaved at 121 °C for 20 minutes. Stability was evaluated based on the following parameters: appearance, pH, kinematic viscosity, and sol-gel transition temperature.
Results and discussion. Autoclaving of various combinations of poloxamers had a negligible effect on the stability parameters of the formulations. The addition of EDTA at high concentrations led to an increase in viscosity, as well as a decrease in pH and gel-forming ability of the formulations. After sterilization, gel precipitation was observed in all samples containing EDTA, but the original appearance of the formulations was restored within 5 days. The other parameters remained stable after autoclaving. The addition of xylitol had a negligible effect on the initial properties of the poloxamers, and the formulations retained stability after sterilization.
Conclusion. The results of the experiments showed that autoclaving is a suitable method for sterilization of systems based on various combinations of poloxamers. The addition of EDTA, especially at high concentrations, should be avoided due to its negative impact on the key parameters of in situ systems and the risk of gel precipitation during autoclaving. Xylitol does not affect the stability of poloxamers during sterilization. However, further research is needed to evaluate the potential of EDTA and xylitol as protective agents for the stabilization of other stimuli-sensitive systems.
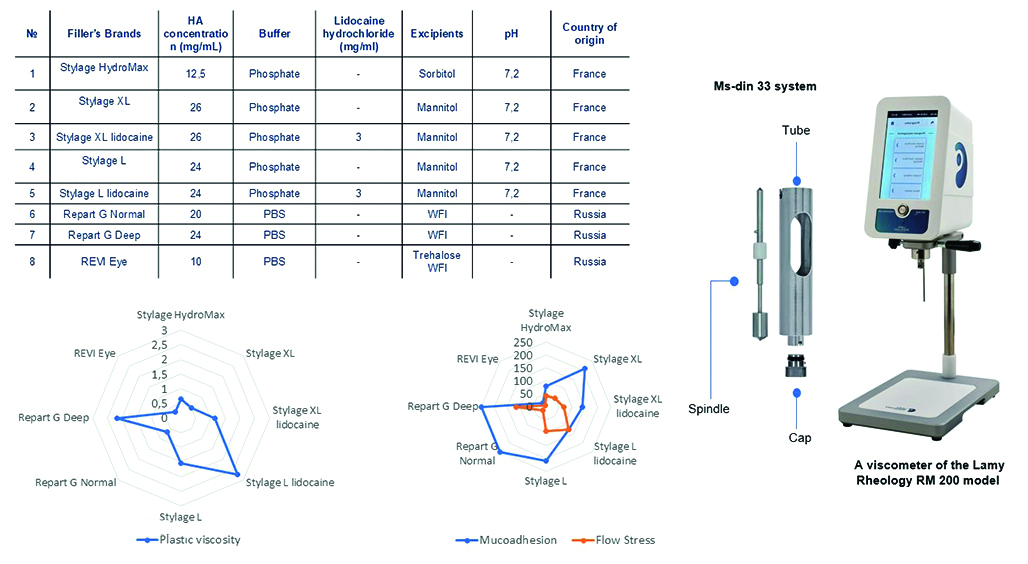
Introduction. Hyaluronic acid (HA) is a polysaccharide belonging to glycosaminoglycans. Due to its high biocompatibility and ability to retain moisture, HA is widely used in cosmetology and medicine. The popularity of HA-based gels is growing, especially in injection cosmetology for the correction of age-related changes, making up a significant part of aesthetic procedures. As a result of the departure of foreign manufacturers from the Russian market, there is a need to develop domestic analogues. The study of the rheological properties of gels helps to optimize formulations for medical use, ensuring the effectiveness and safety of new products.
Aim. To study how the concentration of HA and excipients affect the rheological properties of gels. The study is also aimed at identifying the relationship between these properties and clinical effectiveness.
Materials and methods. Objects of study: fillers of the Stylage® line – Stylage® HydroMax, Stylage® L, Stylage® L lidocaine, Stylage® XL, Stylage® XL lidocaine, and Repаrt® Ġ line – Repart G Normal, Repart G Deep. The samples were examined by rotational viscometry.
Results and discussion. We established a relationship between the clinical characteristics of liquid implants and their rheological properties. In the course of the studies, we determined the following optimal ranges: mucoadhesion – from 100 to 200 Pa, plastic viscosity – from 0,2 to 1,0 Pa ∙ s at a shear rate of 0–100 s–1 at a temperature of 25 ± 1 °C, flow stress – from 50 to 150 Pa at a shear rate of 0–100 s–1 at a temperature of 25 ± 1 °C, thixotropy – complete.
Conclusion. As a result, the study showed that viscosity is important for injection and distribution of the drug in tissues. High mucoadhesion in gynecology and urology determines the fixation of the implant in the tissues. However, excessive mucoadhesion can cause the formation of subcutaneous agglomerates, which limits the use of such products on the face. The relationship between the flow stress and the quality characteristics of the implants was not established.
Introduction. Hepatoprotectors are one of the broad drug groups used in complex therapy at different stages of liver damage. In clinical practice, hepatoprotective agents are used in form of individual or combined drugs. A combined drug is defined as a preparation containing two or more active biological substances that act together in the human body to prevent and treat diseases or to restore and maintain health. Combined drugs are used to increase the treatment effectiveness, reduce side effects, simplify medication intake, or help to treat multiple symptoms at the same time. Often the following well-known hepatotropic agents are most studied and used in combinations: essential phospholipids, glycyrrhizic acid, ursodeoxycholic acid and silymarin. It should be noted that although the pharmacological action of ursodeoxycholic acid and milk thistle preparations has been well studied, no technological research has been conducted to create combined medicines based on them.
Aim. Development of a technology for a combined hepatoprotective agent in form of solid enteric-soluble gelatin capsules with granules containing ursodeoxycholic acid and milk thistle dry extract in form of a solid dispersion system as active substances.
Material and methods. Ursodeoxycholic acid and the solid dispersion system of milk thistle dry extract are included as substances in the formulation of the combined hepatoprotective drug. Solid intestinal-soluble gelatin capsules were filled with granules containing a solid dispersion system of milk thistle dry extract and ursodeoxycholic acid using a ProFiller 3600 desktop manual capsule filling machine, capsule size "2". 3 series of solid gelatin capsules with granules were developed. The capsules standardization was carried out in accordance with the State Pharmacopoeia of the Russian Federation XV edition. The following indicators were determined: description, authenticity, quantitative content of the amount of flavolignans in terms of silybin, quantitative content of ursodeoxycholic acid, mass uniformity of dosage forms, disintegration and dissolution.
Results and discussion. The granules formulation was developed by wet granulation, microcrystalline cellulose as disintegrator, magnesium stearate as lubricant, and 5 % aqueous solution of potato starch were used as excipients. The obtained formulations were compared in terms of quality and technological properties. It was found that granules containing microcrystalline cellulose have the best technological properties, such as weight loss during drying, hygroscopicity, bulk density, Carr index, Hausner coefficient, flowability. For capsules with granules, quality indicators were determined and standardized according to the POA (pharmacopeia official article) "Capsules" in terms of quality indicators: description, authenticity, quantitative content of the amount of flavonoids in terms of silybin, quantitative content of ursodeoxycholic acid, mass uniformity of dosage forms, disintegration and dissolution. When performing the "Dissolution" test, the release of silybin and UDCA was determined in 30, 45 and 60 minutes. The release of silybin was monitored by spectroscopy at a wavelength of λ = 289 nm and the release of ursodeoxycholic acid by high–performance liquid chromatography (HPLC) in accordance with the US pharmacopoeia, USP 44 – NF 39. It was determined that 89.0 ± 0.5 % of silybin was released in 45 minutes, during which time 97.0 ± 0.3 % of ursodeoxycholic acid was released.
Conclusion. The granules formulation with a solid dispersion system of milk thistle dry extract and ursodeoxycholic acid has been developed. Microcrystalline cellulose is used as disintegrant, magnesium stearate as lubricant, and 5% aqueous solution of potato starch as binder. The technological properties of the granules and quality indicators are determined. The technology of intestinal-soluble capsules filled with granules has been developed. The quality indicators were determined and standardized according to quality indicators: description, authenticity, quantitative content of the amount of flavonoids in terms of silybin (by UV spectroscopy), quantitative content of ursodeoxycholic acid (by HPLC), mass uniformity of dosage forms, disintegration and dissolution. The releasing profiles of the active pharmaceutical substance from enteric capsules were studied and it was found that 89.0 ± 0.5 % of the amount of flavolignans in terms of silybin and 97.0 ± 0.3 % of ursodeoxycholic acid were released by 45 minutes, respectively, which meets the requirements of the State Pharmacopoeia of the Russian Federation XV edition.
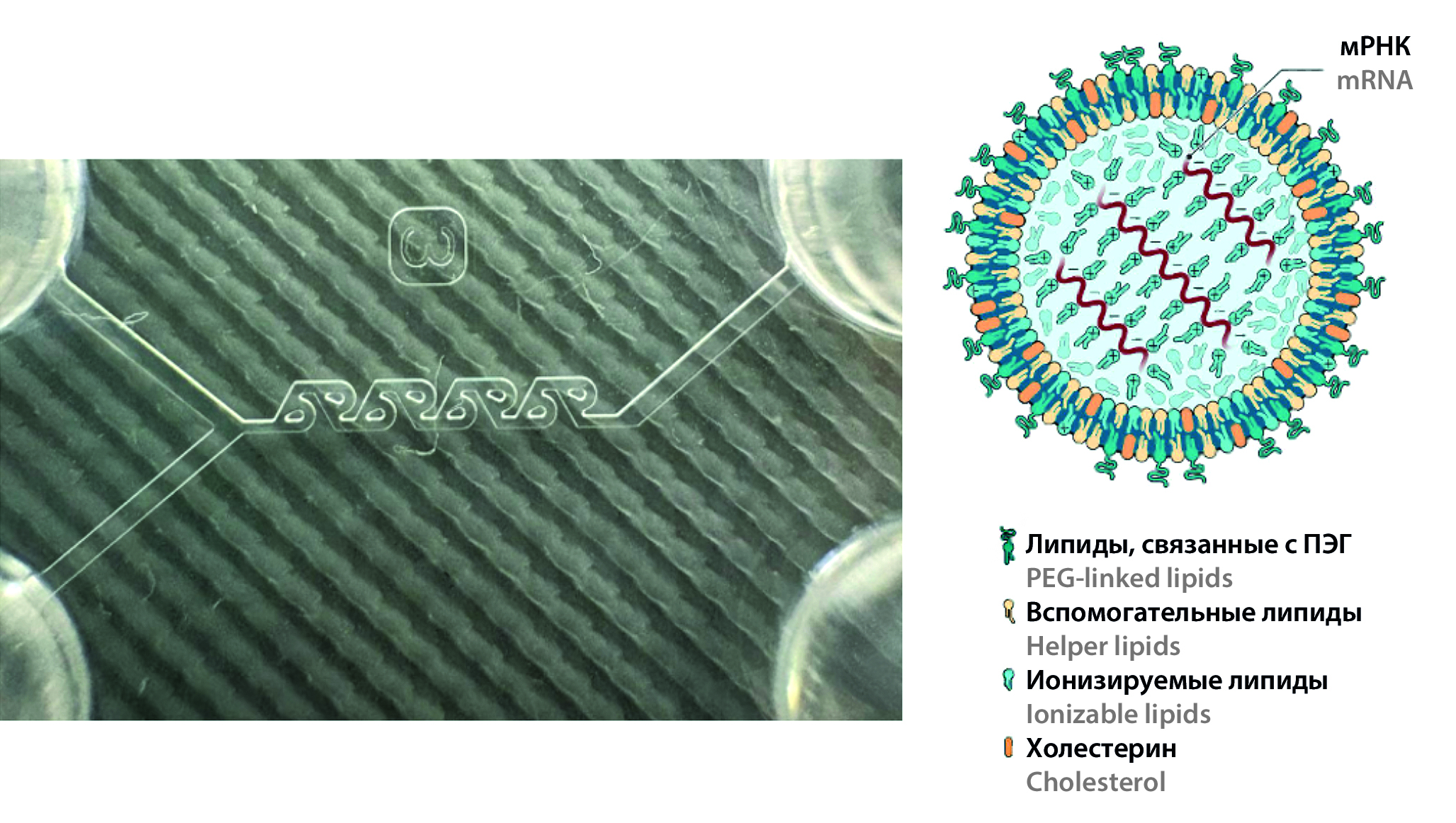
Introduction. Gene therapy is actively developing through the use of mRNA agents for the treatment and prevention of various diseases. To manifest a therapeutic effect, it is necessary to deliver mRNA to target cells and induce the synthesis of target proteins. Key challenges include developing safe and efficient delivery systems. The critical quality attributes for lipid nanoparticles are average particle size, polydispersity index, and ζ potential.
Aim. To study and optimize the assembly conditions of lipid nanoparticles to control their basic characteristics.
Materials and methods. Ionizable lipid heptadecan-9yl(Z)-N-((4-dimethylamino)butyl)thio)carbonyl)-N-(2-(non-2-en-1-yloxy)-2-oxoethyl)glycinate (IL) and helper lipids – dipalmitoylglierophosphate (DPPC), cholesterol, and a-(3"-[1,2-di (myristyloxy) propanoxy]carbonylamino}propyl)-w-methoxypolyoxyethylene (DMG-PEG2000). Solvents: absolute alcohol, purified water. Buffer solutions: acetate buffer solution (pH 4.5), phosphate buffer solution (pH 7.4). Equipment: microfluidic installation Dolomite (Dolomite Microfluidics, Великобритания), Y-shaped polymer microfluidic chip with passive micromixer type "tesla coil", Nanosizer Zeta Pro nanoscale particle analyzer (LLC "Microtrack", Russia).
Results and discussion. The study examined the effects of TFR and FRR parameters on LNF properties. Characteristics such as hydrodynamic diameter (Z-average), average particle diameter (D50), polydispersity index (PDI) and ζ potential were analyzed. The study confirms that with increasing FRR, the average hydrodynamic and median particle sizes decrease. Increasing TFR also decreases Z-average and D50 through hydrodynamic focusing, but at TFR = 3200 μl/min, particle size increases due to decreased nanoemulsion stability and fine particle aggregation. The study did not reveal a direct relationship between FRR and PDI. Nanoemulsions with FRRs of 1 : 3 and 1 : 4 showed the greatest homogeneity. As TFR increases, the polydispersity of the nanoparticles decreases. In the study, the dependence of the ζ potential of lipid nanoparticles on the estimated process parameters was not found. However, as TFR increases, the ζ potential tends to increase.
Conclusion. In the study, the conditions for assembling lipid nanoparticles (LNP) using the microfluidic method were optimized. Mathematical models are proposed to control characteristics of nanoparticles. Optimal conditions for producing LNP: FRR = 1 : 3; 1 : 4; TFR 2000 to 3000 µL/min. If FRR = 1 : 5 and/or TFR > 3000 µL/min are used, the values may be outside the optimal range, which requires additional risk assessment.
Introduction. In vitro equivalence dissolution test is one of the main tests in drug development. In vitro equivalence dissolution test models the bioavailability of the active substance under the conditions of its use, allowing to assess the bioequivalence of drugs. The article describes an approach in which the results of in vitro equivalence dissolution test are used as a basis for obtaining a drug that differs in pharmaceutical dosage form and composition from the referent drug.
Aim. To select a composition of vaginal tablets that would be equivalent to suppositories on a lipophilic base according to in vitro equivalence dissolution test.
Materials and methods. The objects of the study are vaginal tablets and suppositories on a lipophilic base containing natamycin. The study was conducted using dissolution tester (the "Basket" apparatus) and a UV spectrophotometer to calculate the amount of released natamycin.
Results and discussion. The authors proposed an approach in which the development of a new dosage form of natamycin (vaginal tablets) is based on the development of similar to lipophilic base vaginal suppositories registered in the EAEU release profiles. For carrying out in vitro equivalence dissolution test, the authors developed samples of vaginal tablet formulations. The composition was selected to obtain a set of results with different release profiles and select the most optimal one both in terms of profile equivalence with the reference drug and the technological effectiveness (ease of introduction into production and cost-effectiveness of drug production) of the resulting composition. The formulations were selected to provide a set of release profiles that could be further compared with the reference. The objects of study were analyzed using a «Dissolution» test («Basket» apparatus), the released natamycin was monitored by UV spectrophotometry.
Conclusion. In vitro equivalence dissolution test was carried out for vaginal tablets containing natamycin, and the most promising formulation that meets the stated requirements for equivalence of release profiles was selected.
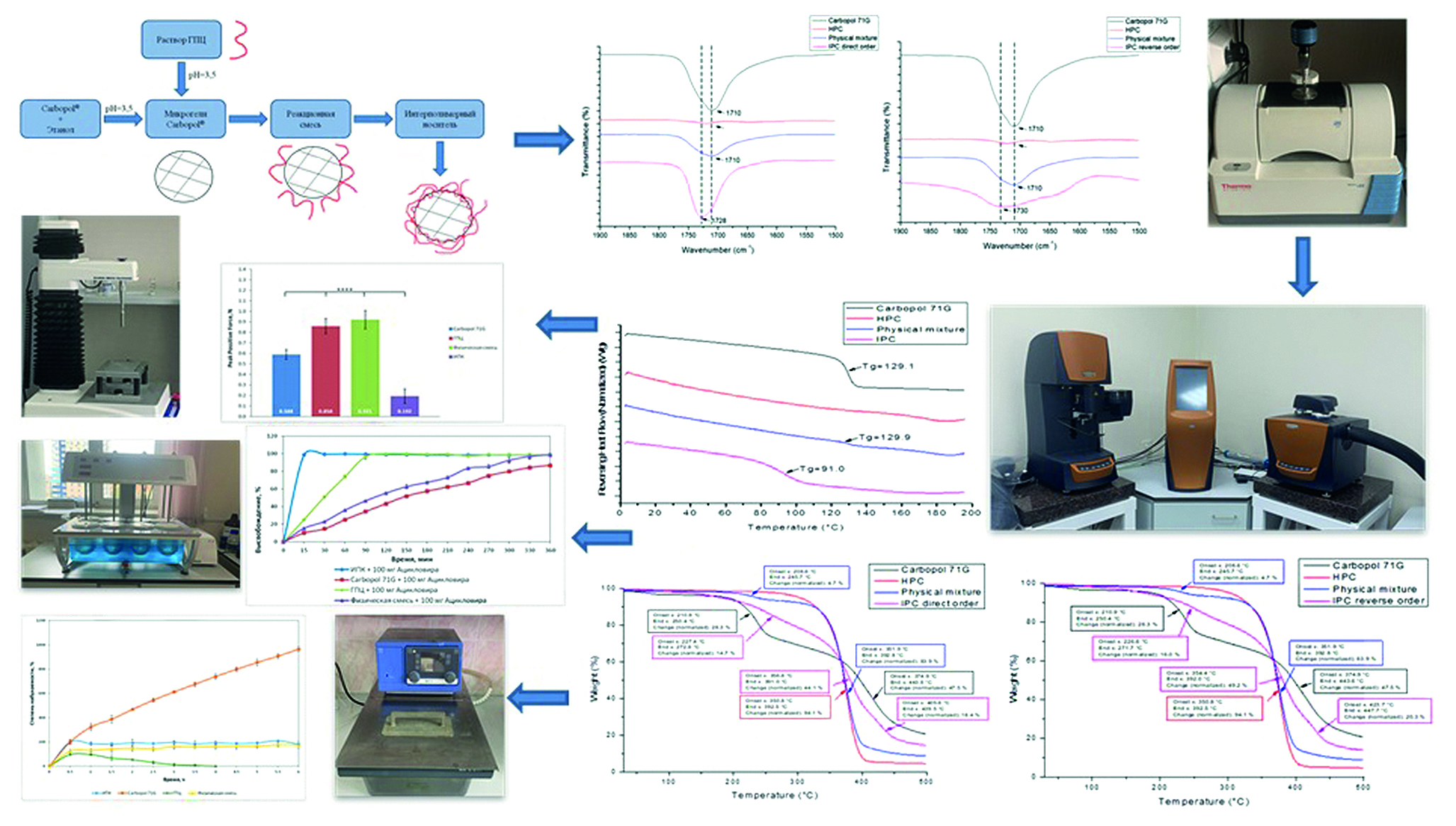
Introduction. As a result of the study, the process of formation of interpolymer complex (IPC) between pairs of polymers was studied between hydroxypropyl cellulose (HPC) and different brands of Carbopol® 71G, 971, 974 at two mixing orders in 95 % ethanol at pH = 3,5 (acidified with 0,1 M HCl) by turbidimetry, IR-spectroscopy, thermogravimetric analysis (TGA). The performed experiments confirmed the formation of an interpolymer complex (IPC) between these pairs of polymers. According to the research results, the prospective ratio of a pair of HPC and Carbopol® 71G was selected in a stoichiometric equimolar ratio regardless of the mixing order. The compatibility of the studied polymers in the composition of the resulting polycomplex was confirmed, using the method of modulated differential scanning calorimetry (mDSC). The obtained IPC had a stoichiometric composition of Carbopol® 71G / HPC 2 : 1 (by moles), according to the elemental analysis. Swelling of matrices based on the synthesized IPC, as well as matrices from individual polymers and their physical mixture (PM), was carried out in a medium simulating stomach acid in comparison with the original polymers. The analysis of the of acyclovir release from the produced carriers was also carried out in a 0.1 M HCl media and showed the prospects of the developed system for creating a carrier with targeted release of drugs into a model environment simulating an empty stomach. The IPC samples showed low mucoadhesive properties compared to individual polymers and their physical mixture. The development of new carriers for drug delivery is one of the key areas of pharmaceutical technology. In this regard, special attention is paid to polymeric substances, carriers based on which provide a reduction in side effects, increased bioavailability and prolongation of the drug action. Gastroretentive delivery systems are of interest in the development of new dosage forms that allow regulating the rate of release of the active pharmaceutical ingredient (API) in the stomach.
Aim. Development of a polycomplex carrier based on hydroxypropyl cellulose with Carbopol® for gastroretentive delivery of acyclovir.
Materials and methods. The selection of IPC – formation conditions was carried out using the methods of turbidimetry, IR-spectroscopy, and TGA. The obtained optimal polycomplex carrier composition was characterized using modulated differential scanning calorimetry (mDSC) and IR-spectroscopy. The swelling of the matrices based on the synthesized polycomplex was studied in an environment that imitating stomach acid. The acyclovir release from the resulting matrices was studied in modeling media using method 1 (USP I) "basket method". Mucoadhesion was studied using a TA.XTplus texture analyzer (Stable Micro Systems, Surrey, UK) on mucin compacts.
Results and discussion. The formation of IPC occurs through the formation of hydrogen bonds between the —OH groups of macromolecular units of linear HPC and —COOH groups of rare-crosslinked polyacrylic acid (rPAA) in the composition of the used brands of Carbopol’s. The leftward shift of the characteristic band to 1730 cm–1 in the FTIR spectra of the polycomplexes confirms the formation of IPCs. The IPCs are characterised by a single glass transition temperature (Tg = 91,0 ± 2,1 ºC). Elemental analysis revealed a two-fold molar excess of the rare cross-linked polymer (Carbopol® 71G) over the linear one (HPC). Throughout the entire experiment to study the kinetics of swelling, the compacted matrices retain their shape, increasing in size. Monitoring of possible structural transformations was carried out, using thermal analysis of samples of polycomplex matrices in the process of assessing their swelling, to confirm the stability of the polycomplex in an acidic environment. According to the results of the model API release kinetics, the maximum concentration of acyclovir released into the media is observed at 30 minutes of the experiment and equal 98.5 %. Whereas for HPC matrices, the maximum concentration is achieved after 2 hours, and for matrices based on Carbopol® 71G and their PM only in the final part of the experiment. The synthesized IPC was characterized by low mucoadhesion capacity on mucin compacts compared to individual polymers and the PM.
Conclusion. As a result of the study, optimal conditions for IPC – formation between pairs of polymers HPC and different brands of Carbopol® (71G, 971 and 974) were selected. Turbidimetry, IR-spectroscopy and TGA methods proved the formation of polycomplexes based on HPC and various brands of Carbopol®. The IPC HPC / Carbopol® 71G of stoichiometric composition, confirmed by elemental analysis, was characterized using thermal and spectral methods. The study of the release of acyclovir from the obtained matrices has shown the promise of using the developed systems for oral gastroretentive delivery.
REGULATORY ISSUES
Introduction. The quality assurance of research work is a key factor in scientific discoveries, as well as the emergence of innovative high-margin products on the market. Due to the specific nature of the scientific research field, there are currently no uniform rules for organizing and conducting research that meet the needs of both scientific groups and businesses interested in the commercialization of developments, including drug developments, and individual approaches are only being formed. The set of established approaches and concepts, according to the existing system of good practices (GxP), is called "Good Research Practice" or GRP.
Аim. Generalization of existing global trends in the field of Good Research Practice and description of the implementation of GRP principles using the example of the quality management system of the Sirius University Laboratory Facility.
Materials and methods. The methods of GRP principles implementation in the Laboratory Complex are based on the requirements of ISO 9001 and ICH recommendations in such areas as document and record management, personnel and training management, audit and inspection management, risk management, analysis and evaluation of performance and efficiency.
Results and discussion. The current principles of GRP, formed within the framework of national scientific communities, individual universities and state programs, are analyzed. Using the example of the Laboratory Facility of the Sirius University, the possibilities and examples of complex implementation of GRP in such areas of the quality management system as document and record management, personnel and training management, audit and inspection management, risk management, conducting periodic analysis and assessment of the effectiveness and efficiency of activities are considered.
Conclusion. The approach to conducting scientific research work described in the article, based on the integrated implementation of GRP, is a tool for ensuring the quality of research, necessary both for purely scientific activities and for successful scientific and applied interaction between the academic environment and pharmaceutical companies in order to accelerate the launch of new drugs to the market and meet patient demand.
ANNIVERSARY
ISSN 2658-5049 (Online)