FROM EDITOR
The Congress "Chemical and Biological Drugs: Pharmaceutical and Clinical Development in Accordance with the EAEU rules" will be held in Moscow on September 22. We will discuss such themes as pre-registration trials which are necessary for quality, effectiveness and safety assurance. As well as we will discuss technology transfer and post-authorisation studies of drugs.
The first part of the article, based on a systematic analysis of the decrees of Peter the Great, presents the steps of the Russian Emperor to establish and develop the pharmacy service in Russia are given. An analysis of the text of the decrees shows that Peter the Great's special interest in the distribution of medicines appeared from the end of the 17th to the beginning of the 18th century, after his first visit to Europe. His first step, aimed at controlling the distribution of medicines, was the Decree to the customs service on the need to inspect and send to Moscow pharmaceutical supplies brought from abroad. Having gained control over the import of medicines and their monopoly distribution, Peter the Great organized the delivery of medicines from the royal pharmacy to the army. The Emperor’s next step was the foundation of a network of Moscow pharmacies. To do this, he issued a Decree on the establishment of eight pharmacies in Moscow. Later, such pharmacies were also established in St. Petersburg and other major Russian cities. Peter the Great gave the owners of these pharmacies nominal letters designed to protect them from excessive extortion and injustice of the city authorities. It was necessary to establish the supply of the new pharmacies with the appropriate raw materials. Peter the Great, by his decrees, ordered to purchase medicinal herbs collected in Siberia, to create pharmacy gardens in Moscow, St. Petersburg and Astrakhan, and to restrict trade in certain important goods. In addition, according to the decree of Peter the Great, scientific expeditions are sent around Russia to search for mineral water and explore the riches of Siberia.
EVENTS
The 21st International Exhibition of Laboratory Equipment and Chemical Reagents Analitika Expo was held at the Crocus Expo from the 11th till 14th of April.
RESEARCH AND DEVELOPMENT OF NEW DRUG PRODUCTS
Introduction. Xanthone glycosides have unique structures and properties. Many efforts focus on the search for C-glycoside derivatives of mangiferin with higher bioavailability. The application of the QSAR approach allows for the optimization of the search for novel xanthone derivatives with the desired characteristics.
Aim. Using available descriptors of chemical structure, physical-chemical properties, and biological activity, analyze a sample set of known homologs and analogs of mangiferin to QSAR prognosis bioactivity of new xanthone C-glycosides.
Materials and methods. 26 molecules of natural homologs and modified derivatives of mangiferin formed the analyzed sample set. Topological graphs of compounds were constructed using ChemicPen software. ChemicDescript software was used for the calculation of molecular descriptors, including the Balaban index. Physicochemical characteristics of molecules as well as Lipinski's rule criteria were calculated in Molinspiration. The spectrum of the most probable (Pa > 0.7) biological activity of the described compounds were predicted using Pass Online. The software Origin (OriginLab, USA) was used for the graphical representation of the results.
Results and discussion. Mangiferin and its natural homologs are the most hydrophilic compounds. The hydrolysis of the C-glycosidic bond, alkylation, acylation, and the introduction of an amino substituent radical into the mangiferin structure led to the increase of its lipophilic properties. The spectrum of the most probable biological activities of the described molecules: antitumor, antioxidant, and cardioprotective effects. The results of ADMET modeling based on the substance-drug similarity criteria showed that only 4 compounds correspond to the rule of five. We proposed the validation model to predict bioactivity from lipophilicity and molecule structure described with Balaban index. The error of prediction obtained in a result of cross-validation turned out to be about less than 3 %.
Conclusion. A correlation between the structure and properties of the molecules discussed has been demonstrated. The obtained results can be used for further prediction of the properties of natural and synthetic xanthone C-glycosides and directed synthesis of new active compounds.
Introduction. Due to the rapid development of nanotechnology, selenium nanoparticles (NPs) have recently attracted much attention due to their unique physical and chemical properties for biomedical applications, in particular for the treatment of oncological diseases. The review considers the selenium nanoparticles, which are widely studied in the field of oncology.
Text. This review is devoted to the analysis of scientific literature on the anticancer activity of selenium nanoparticles against human cancer cell lines, as well as the application of these nanoparticles as a drug delivery system. Besides, the antitumor mechanisms of selenium nanoparticles against malignant neoplasms are discussed.
Conclusion. According to the results of literary data analysis, it was found that selenium nanoparticles exhibit a good antitumor effect against various human cancer cell lines. It is shown that the antitumor activity of selenium nanoparticles is mainly related to activation of the extrinsic and intrinsic signaling pathways of apoptosis leading to cancer cell death. Also, selenium nanoparticles are promising systems for delivery of various anticancer drugs, providing high efficiency, bioavailability of drugs in tumor cells and minimizing toxicity to healthy cells.
Introduction. The search for new, effective and safe pharmacologically active substances remains an urgent task in the field of pharmacy. Many compounds of the piperidine and morpholine series are widely used in medical practice and belong to an important group of biologically active compounds. An informational, literature search on the synthesis of new derivatives of piperidine and morpholine was carried out. The article summarizes the results of studies of new derivatives of piperidine and morpholine as potential sources of biologically active substances.
Text. The review is devoted to the relationship between the pharmacological activity of the N-derivatives of piperidine and morpholine in relation to various biological targets and the structure of the substance, the importance of the piperidine and morpholine rings in the design and development of drugs is highlighted. Piperidine and morpholine are considered as prerogative structures not only for increasing activity, but also for obtaining biological substances with desired therapeutic properties and improved pharmacokinetics.
Conclusion. The literature review shows the current trend towards the study of morpholine and piperidine derivatives, reveals their high pharmacophore activity. The review will provide researchers with the necessary knowledge base to make chemical structural changes to the structures of drug leaders to enhance pharmacological activities.
PHARMACEUTICAL TECHNOLOGY
Introduction. Excipients, impurities contained in them, and sorbed water are one of the reasons for degradation of the active pharmaceutical substance (API). Excipients effect should be especially evaluated for moisture-sensitive APIs. Folic acid (FA) is an important vitamin for humans. It hydrolyze in water under the action of UV irradiation and main decomposition product is N-(p-aminobenzoyl)glutamic acid (impurity A). We found an increase in the content of impurity A during FA film-coated tablets storage in PVC-film and aluminum foil packaging in the absence of UV irradiation.
Aim. Investigate the effect of excipients and parameters of the production process on the content of impurity A during storage of FA drugs.
Materials and methods. The FA tablets containing 1.0 mg of API produced by direct compression technology were the objects of study. The pressing force (PF) was varied from 5 to 15 kN.
Results and discussion. We found that content of impurity A in tablets containing 93.0 % lactose monohydrate and obtained with PF above 10 kN exceeded limit value during storage for 300 days. Probably lactose simultaneously acts both as a source of free water and as a catalyst for FA hydrolysis. Since the interaction of lactose and FA occurs in the solid phase, pressing accelerates hydrolysis by increasing the contact area of substances and the mobility of water molecules.
Conclusion. We found that lactose monohydrate probably is the main cause of FA hydrolysis in drugs. Independently of the mechanism of its action, an increase in the PF above 10 kN leads to an increase in the rate of FA hydrolysis. This is due to an increase in the mobility of water molecules and the contact area between the excipient and API. We have selected the optimum pressure range (5–10 kN) for tablet mix containing lactose monohydrate and FA.
ANALYTICAL METHODS
Introduction. Taurine is a non-proteinogenic amino acid. The molecule is involved in lipid metabolism, adsorbs fat-soluble vitamins, and its conjugates with bile acids contribute to the emulsification of fats in the intestine. Drugs, which include a taurine molecule, have anti-cataract, cardiotonic, metabolic effects, and also stimulate regeneration. Among the dosage forms, where taurine acts as an active substance, there is a solid dosage form – film-coated tablets. One of the methods for assessing the quality of solid dosage forms is a comparative dissolution kinetics test. High-performance chromatography with ultraviolet detection is a widely used method for quantification within the dissolution test, however, for taurine, which does not contain chromophore groups in its structure, this method is not directly applicable. To solve this problem, one can apply the method of pre-column derivatization, because of which an fragment is introduced into the structure, providing a bathochromic shift in the UV spectrum of the starting compound.
Aim. Development, validation and approbation analytical method for the quantitative determination of taurine by high-performance chromatography with ultraviolet detection as part of a test comparative kinetics dissolution of taurine tablets with a dosage of 250 and 500 mg.
Materials and methods. The following preparations were used for the analysis: taurine tablets, film-coated 250 mg and 500 mg, domestic production with a valid expiration date. The comparative dissolution kinetics test was carried out on a DT 126 Light instrument for the "Dissolution" test (ERWEKA GmbH, Germany). Chromatographic separation and detection were performed on a Nexera-i LC-2040 high-performance liquid chromatograph (Shimadzu Corporation, Japan) equipped with a column and sample thermostat, a degasser, an autosampler, and an ultraviolet detector. Detection was carried out at a wavelength of 254 nm after derivatization of the taurine molecule with 4-toluenesulfonyl chloride. Were used a Shim-pack Velox C18 5 μm 4.6 × 150 mm column (Shimadzu Corporation, Japan) and a Shim-pack Velox C18 EXP Guard Column Cartridge 5 μm 4.6 × 5 mm (Shimadzu Corporation, Japan). Primary data were processed using LabSolutions Single LC software (Shimadzu Corporation, Japan).
Results and discussion. The optimal conditions for taurine derivatization have been selected, and a method for the quantitative determination of taurine by HPLC-UV in test comparative kinetics dissolution in three dissolution media: 0.1 M hydrochloric acid solution with pH 1.2, acetate buffer solution with pH 4.5, phosphate buffer solution with pH 6.8, as well as in the quality control medium – purified water has been developed and validated. During the validation of the developed methodology, it was found that the validation characteristics are within the acceptance criteria in 4 dissolution media. The analytical range of the method was 0.05–1.2 mg/mL and allows the developed method to be used for the quantitative determination of tablets with a dosage of 250 mg and 500 mg as part of the test comparative kinetics dissolution. The method was tested in 4 dissolution media, in all media, there was a complete release in both dosages (more than 85 % by 30 minutes).
Conclusion. The method was tested in three dissolution media: 0.1 M hydrochloric acid solution with pH 1.2, acetate buffer solution with pH 4.5, phosphate buffer solution with pH 6.8, as well as in the quality control medium – purified water. In all media, there was a complete release in both dosages (more than 85 % by 30 minutes).
Introduction. Golden dock (Rumex maritimus L., Polygonaceae) is used as a medicinal and food plant in Asian countries. The plant contains phytochemicals of various classes: flavonoids, tannins, anthraquinones etc. Plant extracts exhibit antibacterial, antioxidant, anti-inflammatory, astringent activity, and have antidiabetic potential. The plant is annual, and most of the biologically active substances accumulate in its aboveground organs. An important problem is the standardization of Rumex maritimus and the development of regulatory documentation for its the introduction to medical practice.
Aim. To conduct phytochemical analysis of the aerial part of golden dock.
Materials and methods. Air-dried aerial part of Rumex maritimus collected at flowering and beginning of fruiting stage, as well as individual above-ground organs (leaves, flowers, fruits, stems), were used for obtaining the extracts. Qualitative analysis of the extracts was carried out using reverse phase HPLC. The relative content of the components in the mixture was calculated by the method of simple normalization. Total content of free anthraquinones and anthraglycosides in terms of chrysophanic acid was determined using spectrophotometric method after acid hydrolysis. Total tannin content was calculated by titrimetric method.
Results and discussion. Flavonoids isoquercetin and avicularin were first discovered in the aerial part of Rumex maritimus. The dominant component of the plant is rutin. Chrysophanol predominates among anthraquinones. The highest concentration of anthraquinones (2.80 ± 0.04 %) was found in flowers. Tannins accumulate mainly in leaves (9.97 ± 0.02 %). A significant amount of tannins (6.60 ± 0.03 %) and anthracene derivatives (1.96 ± 0.03 %) is contained in the whole aerial part.
Conclusion. Phytochemical analysis of the aerial part of Rumex maritimus showed the presence of a significant amount of anthraquinones. As a plant raw material it is proposed to use the herb of Rumex maritimus. Standardization is recommended for anthraquinones in terms of chrysophanic acid (at least 1.5 %).
Introduction. Scanning electron microscopy is a modern method that allows us to study not only the morphological features of objects, but also to conduct micro-X-ray structural analysis. Currently, the method is being actively introduced into the study of biological objects (including plant ones). Sea buckthorn (Hippophaë rhamnoides L.) leaves are a non-pharmacopoeial type of medicinal plant raw materials. Further study of the morphology, anatomy and phytochemical composition of leaves can contribute to the production of new, including combined, medicines, which will require the development of a pharmacopoeia article for this medicinal plant raw material.
Aim. The aim of the study was to study the morphological and anatomical features of sea buckthorn leaves by scanning electron microscopy.
Materials and methods. The object of the study was dried whole leaves of sea buckthorn (Hippophaë rhamnoides L.), collected in the Voronezh region in 2021 during the period of mass fruit maturity. To carry out the study by scanning electron microscopy, pieces of leaves were previously sprayed with gold on an automatic spraying unit Q150R ES (Quorum Technologies Ltd., United Kingdom) to increase conductivity. Micrographs were obtained using an electron microscope JSM-6510LV (JEOL Ltd., Japan).
Results and their discussion. The morphology and some features of the anatomical structure of sea buckthorn leaves were studied and the main microdiagnostic signs (surface character, types of trichomes, the presence of stomata) were clarified. The content of elements (silicon, potassium, aluminum, carbon and calcium) was determined during the microrentgenostructural analysis. Micrographs of pollen grains of the plant were obtained, the presence of the element iron in them was established.
Conclusion. For the first time, the method of scanning electron microscopy was used to study the morphological and anatomical features of sea buckthorn leaves. The main diagnostic signs of leaves and their location have been clarified. It has been established that carbon predominates in the composition of the raw material elements, and calcium also accumulates. The accumulation of aluminum, silicon and potassium in numerous hairs densely covering the upper, and especially the lower, surfaces of the leaf blade is assumed. The morphology of the surface of pollen grains of sea buckthorn, having a spherical shape with a spiny shell, has been established. Pollen grains, in addition to carbon, are characterized by the accumulation of iron and silicon.
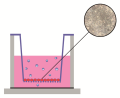
Introduction. Breast cancer resistance protein (BCRP) is an efflux membrane transporter that controls the pharmacokinetics of a large number of drugs. Its activity may change when taking some endo- and exogenous substances, thus making it a link in drug interactions.
Aim. The aim of the study was to develop a method for testing of drugs for belonging to BCRP substrates and inhibitors in vitro.
Materials and methods. The work was performed on Caco-2 cells overexpressing BCRP, the cultivation was performed in a transwell-system consisting of the apical and basolateral chambers. Cells were seeded at the bottom of the apical chamber, which is a semipermeable membrane. Primarily, the transport of BCRP substrates: methotrexate, mitoxantrone and quercetin was evaluated in the concentration range of 1, 5, 10, and 50 μM in the direction from the basal chamber to the apical one (Papp b-a) and in the opposite direction (Papp a-b). The ratio Papp b-a / Papp a-b more than «2» characterizes the participation of transporter proteins in the transcellular transport of substances. To confirm the participation of BCRP in their transport the experiment was carried out with the addition of a transporter inhibitor, reserpine, to the transport medium at a concentration of 50 μM. The concentration of substrates in the chambers was analyzed by HPLC-MS/MS.
Results and their discussion. The addition of methotrexate (1 μM), mitoxantrone (1 μM), and quercetin (1–10 μM) to both the apical or basolateral chambers of the transwell-system, their content in the recipient chamber was not detected. When methotrexate concentration became 5 μM the Papp b-a / Papp a-b ratio was 3.38 ± 0.08, which indicates the involvement of transporters in its transfer. The addition of methotrexate to the donor chamber at concentrations of 10 and 50 μM, Papp b-a / Papp a-b decreased to values below «2». At mitoxantrone concentration of 5 μM Papp b-a / Papp a-b was 2.72 ± 0.16. An increase in the concentration to 10 μM led to an increase in Papp b-a / Papp a-b to 6.18 ± 0.08. With a substance content of 50 μM the indicator decreased but remained above the value «2». In the quercetin concentration of 50 microns, Papp b-a / Papp was below "2". Reserpine reduced Papp b-a / Papp a-b of methotrexate by 3.31 times (p = 0.0002), which indicates the elimination of asymmetry in the transport of the substance. At a mitoxantrone concentration of 10 microns, reserpine reduced its Papp b-a / Papp a-b by 3.36 times (p < 0.0001). The results indicate the participation of BCRP in the control of the transfer of both substances through the cellular monolayer.
Conclusion. A method of testing drugs belonging to BCRP substrates and inhibitors using methotrexate (5 μM) and mitoxantrone (10 μM) as marker substrates and reserpine (50 μM) as inhibitor was developed and tested on Caco-2 cells.
Introduction. Etoricoxib is a selective cyclooxygenase (COX-2) inhibitor used for the treatment of acute pain and has anti-inflammatory and analgesic efficacy. Etoricoxib causes fewer complications compared to other non-steroidal anti-inflammatory drugs (NSAIDs). FSI "SID and GP" has developed an ophthalmic liquid dosage form based on etoricoxib. This article proposes a method for determining the content of etoricoxib in a liquid dosage form by high performance liquid chromatography with UV detection.
Aim. Development and validation of a method for the quantitative determination of etoricoxib in liquid dosage form.
Materials and methods. Eye drops with a concentration of the active substance etoricoxib of 0.05 % were used for the analysis, a standard sample of etoricoxib (Kekule Pharma Limited, India, series ACE-3 WS001/15). Chromatographic separation performed on an Agilent 1220 Infinity II LC high performance liquid chromatograph (Agilent Technologies, USA) equipped with a gradient pump, a column thermostat, and a diode array detector. The analysis carried out on a Kromasil C8 column 250 × 4.6 mm, using acetonitrile and 0.05 M buffer solution of potassium dihydrogen phosphate pH = 4.2 as a mobile phase in a ratio of 46 : 54. The analysis time was 15 minutes at a detection wavelength of 235 nm.
Results and discussion. A method for the quantitative determination of etoricoxib in a liquid dosage form developed and validated according to the following indicators: specificity, linearity, accuracy, range, intermediate precision, repeatability.
Conclusion. According to the results of validation tests, all of the listed parameters meet the acceptance criteria. The proposed method characterize by high efficiency and specificity.
Introduction. The formation and accumulation of biologically active substances in plants is a complex process associated with a number of environmental factors, including anthropogenic ones. The study of the characteristics of the qualitative composition of essential oil of wormwood grass of bitter different from the ecological point of view of the places of harvesting is relevant.
Aim. The aim of this study study of the qualitative composition of essential oil of bitter wormwood grass, harvested in areas of the Voronezh region that are different from an ecological point of view.
Materials and methods. In the Voronezh region, 4 points of raw materials procurement were selected, diverse in terms of anthropogenic impact. Isolation of essential oil from the raw materials was carried out according to the method of PS "Wormwood of bitter grass". Component composition of the obtained essential oils was determined using Agilent 7890B GC System (Agilent Technologies, USA) with Agilent 5977A MSD mass selective detector (Agilent Technologies, USA). Data analysis and processing was carried out on the basis of NIST11 databases (from 19.05.2011), MassHunter ver. B.06.00 and NIST MS Search ver. 2.0 software were used.
Results and discussion. In a sample of essential oil obtained from reserved raw materials, monoterpene compounds account for more than 82 %, and sesquiterpene compounds – 16.6 %, about 2 % are organic impurities. Intensive biosynthesis of monoterpenes was noted in a sample collected in the area of agricultural fields of the Verkhnekhavsky district, its mass fraction is more than 73 %. In samples of raw materials with anthropogenic load (OJSC "Minudobreniya" and Highway M4), the share of monoterpene compounds is significantly lower (63.7 and 49 %, respectively). The increase in the proportion of sesquiterpene compounds in the last samples of wormwood essential oil may be due to the excessive acidity of the urbanized places of the workpiece. The sesquiterpene compound hamazulene, which stains essential oil blue, is identified in only two samples of gorse grass wormwood harvested along agricultural fields and in M4 tracks, which explains the presence of a blue hue in these oils.
Conclusion. The chromato-mass spectrometric analysis of the essential oil of the studied samples of bitter wormwood grass made it possible to identify more than 70 different components in them, while the qualitative composition of the essential oil of the raw materials of different places of the workpiece was significantly different, which may indicate a significant influence of the place of growth of the species and anthropogenic factors on the features of the secondary metabolism of terpene compounds in the plant organism.
Introduction. Trace elements are an essential part of the human body, but about 80 % of the human population notes an imbalance in their content. Medicinal plants contain minerals in an accessible and digestible form together with biologically active substances. Medicinal herbal preparations are very popular for the prevention and as part of the complex therapy of various diseases. However, information about the content of trace elements in multicomponent medicinal herbal preparations is very limited. Therefore, it is necessary to study the mineral composition, which will allow us to consider plant collections as an additional source of trace elements.
Aim. The purpose of our study was to study the content of trace elements (B, Si, Al, Ba, Sr, Ti) in breast collection No. 4, its components and aqueous extracts from them.
Materials and methods. The objects of the study were breast collection No. 4, its individual components and aqueous extracts from them. Infusions from the samples were obtained according to the instructions for use on the packaging of a medicinal herbal preparation. The samples were prepared for analysis with a mixture of concentrated nitric acid and water deionized in the Milestone Ethos Up microwave system (Milestone, Italy). The analysis was carried out by atomic emission spectrometry with inductively coupled plasma on the ISP-NPP 720-ES device (Agilent Technologies, USA).
Results and discussion. It was found that the concentrations of Si, Al, B, Sr, Ba, Ti in individual components of the collection varied in the range of 2.9–1240 mg/kg, the transition of trace elements to aqueous extracts was 0.4–34.2 %. The content of these elements in breast collection No. 4 was found to be 13.3–920.7 mg/kg, and the degree of extraction the infusion was 3–40 %. Comparative analysis showed that the extraction of B, Al, Ba from the collection into the infusion is 14–58 % higher than from the individual components included in its composition. It has been established that 50 % of boron and 264 % of silicon from the recommended adequate level of consumption in the Russian Federation enters the human body with an infusion from the breast collection No. 4.
Conclusion. The study showed that breast collection No. 4 can be considered as an additional source of B and Si in the human body. The concentrations of Al, Sr, Ba, Ti were within the average values of the range of the content of these elements in plants.
Introduction. Currently, biosimilars have found quite widespread use in the treatment of a number of chronic and life-threatening diseases. Thanks to them, there is a significant decrease of the economic pressure of biological drugs on the health care system and wide access of patients to effective and safe medicines is ensured. One of the most important stages of proving biosimilarity is the physicochemical and functional characterization of proteins. This set of studies is generally accepted, as sensitive as possible and allows us to give a conclusion about the compliance of the biosimilar with the original drug.
Aim. Conducting physicochemical and functional characterization of medicinal product Rinsulin® R (GP40051) in comparison with the original drug Humulin® Regular.
Materials and methods. Primary structure was analyzed by high-performance liquid chromatography with mass spectrometry detection and matrix assisted laser desorption/ionization. The identity of the higher protein structures was proved by the methods of circular dichroism, capillary isoelectric focusing, spectrometry and dynamic light scattering. The comparability of the impurity profiles of the preparations was evaluated using the methods of exclusive chromatography and reverse-phase high-performance liquid chromatography. Functional characterization included a metabolic cell test "glucose uptake" and insulin receptor–binding assay (kinetics of binding to type A and B receptors, phosphorylation of insulin receptor).
Results and discussion. In the course of this research, the identity of the physicochemical and functional characteristics of GP40051 was shown. A complete overlap of primary sequence, high-order structures and impurity profiles was demonstrated between the comparison drug GP40051 and the reference drug Humulin® Regular. Functional studies have shown that GP40051 and Humulin® Regulars have the same activity.
Conclusion. The results of the quality comparability study demonstrated similarity of Rinsulin® R to the reference medicinal product Humulin® Regular, providing the scientific basis for conducting a specifically designed clinical programme, and supported registration in Russian Federation.
PRECLINICAL AND CLINICAL STUDIES
Introduction. SARS-CoV-2 (severe acute respiratory syndrome-related coronavirus 2) is expected to remain a persistent global threat. Therefore, development of coronavirus disease 2019 (COVID-19) drugs is the most urgent global issue. Nirmatrelvir and ritonavir combination is an oral antiviral drug combination with activity against SARS-CoV-2. Nirmatrelvir and ritonavir combination is highly efficacious in reducing the risk of COVID-19. The study describes development and validation of high-performance liquid chromatography – tandem mass spectrometry (HPLC-MS/MS) method for the simultaneous determination of nirmatrelvir and ritonavir in human blood plasma. The method could be applied in pharmacokinetic study of nirmatrelvir and ritonavir.
Aim. The aim of this study is to develop and validate a HPLC-MS/MS bioanalytical method for the determination of nirmatrelvir and ritonavir in human plasma.
Materials and methods. The determination of nirmatrelvir and ritonavir in human plasma by HPLC-MS/MS. The samples were processed by acetonitrile protein precipitation. Internal standard: promethazine. Mobile phase: 0.1% formic acid solution in water (Eluent A), 0.1% formic acid in acetonitrile (Eluent B). Column: Phenomenex Luna C18 50 × 2.0 mm, 5 μm. Analytical range: 50.00–10000.00 ng/mL for nirmatrelvir, 5.00–1000.00 ng/mL for ritonavir in human plasma. Ionization source and ionization: electrospray ionization, positive. Detection conditions: 499.90 → 110.10 m/z, 499.90 → 319.20 m/z (nirmatrelvir), 720.90 → 426.00 m/z, 720.90 → 296.20 m/z, 720.90 → 268.10 m/z, 720.90 → 197.10 m/z, 720.90 → 139.90 m/z (ritonavir), 285.15 → 198.05 m/z (promethazine).
Results and discussion. This method was validated for selectivity, matrix effect, calibration curve, accuracy, precision, spike recovery, the lower limit of quantification, carry-over effect and stability.
Conclusion. The HPLC-MS/MS method for quantitative determination of nirmatrelvir and ritonavir in human plasma was developed and validated. The analytical range was 50.00–10000.00 ng/mL for nirmatrelvir, 5.00–1000.00 ng/mL for ritonavir in human plasma. This method was applied to investigate the pharmacokinetics of nirmatrelvir and ritonavir.
Introduction. The novel coronavirus infection COVID-19 (Coronavirus Disease 2019) is caused by an enveloped, positive-sense, single-stranded ribonucleic acid (RNA) virus SARS-CoV-2 (severe acute respiratory syndrome-related coronavirus 2). Favipiravir is the antiviral drug recommended for etiotropic treatment of COVID-19. Parenteral therapy has advantages over the other routes of the drug administration: there are no interaction with food and digestive enzymes, may be used for patients with diseases of the digestive system and unconscious patients. For parenteral drug administration of favipiravir the drug "Areplivir" has been registered in Russia.
Aim. The aim is pharmacokinetics study of drug "Areplivir", a lyophilisate for the preparation of a concentrate for the infusion solution (the manufacturer is JSC "Biokhimic", LLC "Promomed RUS" as registration certificate holder) by intravenous infusion in healthy volunteers in a phase I pharmacokinetics study.
Materials and methods. The clinical and analytical phases of the pharmacokinetic study as well as pharmacokinetic analyses have been performed as part of a clinical trial of the drug "Areplivir" in different doses, a lyophilisate for the preparation of a concentrate for the infusion solution (LLC "Promomed RUS", Russia). Chromatographic separation and detection were carried out on a LC-2040C high-performance liquid chromatograph (Shimadzu Corporation, Japan) with a built-in UV detector, a low-pressure four-component gradient pump, a degasser, an autosampler, a column thermostat and a controller. The pharmacokinetic parameters were calculated with the Boomer pharmacokinetic analysis add-in for Microsoft Excel (Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA 92606, USA). Descriptive pharmacokinetic statistics were calculated with Microsoft Excel (Microsoft Corporation, USA). Correlation and Regression Analysis were conducted with IBM SPSS Statistics (version 23.0), IBM, USA.
Results and discussion. For single dose administration of 400, 800, 1600 and 1800 mg in 4 cohorts of 5 volunteers pharmacokinetic parameters were calculated. For Cmax and an administered dose the strong correlation coefficient on the Chaddock scale (r = 0,98; p = 0,02; r – Pearson correlation coefficient; p – the reached significance value) and the determination coefficient (R2 = 0,96; F = 45,97; p = 0,02; R2 – determination coefficient; F – the actual value of the Fisher's criterion) were statistically significant. For AUC0-t and an administered dose the strong correlation coefficient on the Chaddock scale (r = 0,97; p = 0,03) and the determination coefficient (R2 = 0,94; F = 33,54; p = 0,03) were statistically significant. The obtained results show the linearity of Cmax and an administered dose and the linearity of AUC0-t and an administered dose (400–1800 mg).
Conclusion. According to the concentrations of favipiravir from the analytical phase of the pharmacokinetic study the pharmacokinetic parameters were calculated, averaged pharmacokinetic profiles in linear and log-linear scales were plotted after single dose administrations of the drug "Areplivir" in different doses, a lyophilisate for the preparation of a concentrate for the infusion solution (LLC "Promomed RUS", Russia). The linearity of Cmax and a single administered dose and the linearity of AUC0-t and a single administered dose of the drug "Areplivir" have been demonstrated for doses of 400 to 1800 mg. The results justified the study of multiple dose administration of "AREPLIVIR" and the subsequent phases of clinical trials.
Introduction. Pharmaceutical development of an innovative highly effective and competitive drug is a long and expensive process, the result of which is quite difficult to predict in advance. To speed up the entry of a new drug to the treatment and reduce the developer's material costs, it is advisable to include preclinical experiments in the process of creating a drug.
Text. The purpose of this work is to create a justified approach to the implementation of laboratory pharmaceutical development involving in vivo studies. The inclusion of preclinical studies in the process of laboratory pharmaceutical development will eliminate the negative impact of pharmaceutical factors on the bioavailability of a drugs and avoid errors in the selection of excipients, as well as reduce material and time costs. The review presents examples that demonstrate the relevance of conducting preclinical experiments at different stages of pharmaceutical development. These examples made it possible to describe a clearer algorithm of actions in the laboratory pharmaceutical development of a new drug from the moment a drug candidate molecule is selected.
Conclusion. Due to increase the probability of successful pharmaceutical development at initial stage, it is necessary to carry out pharmacokinetic and/or pharmacodynamic experiments to make it possible to develop a drug with an optimal pharmacokinetic profile, reduce the number of preclinical studies, the cost of development, and ensure successful translation of data into clinical practice.
Introduction. A review of the scientific literature showed that the current standards for assessing the quality of drugs does not include an assessment of the osmotic activity of drug solutions and their local irritant effect on tissues at the sites of subcutaneous, intramuscular and intravenous injections. Therefore, currently injectable solutions considered to be of high quality may not have isotonic activity and high postinjection safety.
Text. A study of the concentration range of quality drug solutions ready for injection showed that the acceptable concentration value of the main ingredients is in the range of 0.01 to 76 %. Direct measurement with an osmometer of the osmotic activity of injection solutions, considered qualitative today, has shown that injection solutions can have hypotonic, isotonic and hypertonic activity and their osmotic activity can be in the range of 0 – 3900 mosmol/l water. Study of acidic activity of drug solutions showed that in accordance with pharmacopoeial requirements of drug quality modern quality drug solutions ready for injection can have acidic, neutral or alkaline activity. Solutions with hypertonic activity have been found to have a local irritant effect. Moreover, an increase in hypertonic activity of drug solutions increases their local irritant effect. It has been found that excessively high hypertonic activity of drug solutions may be the cause of the development of a local postinjection complication known as "Nicolaou syndrome", the cause of which has remained unknown for a long time. Nicolaou syndrome includes local pain syndrome, aseptic inflammation, necrosis, and abscess.
Conclusion. The authors conducted a literature review, the results of which led to conclusions and assumptions. Solutions containing drugs in concentrations greater than 10 % may have the highest hypertonic activity, which can cause excessive dehydrating, local irritating and cauterizing effects. Therefore, injections of such drugs are most dangerous with the development of post-injection necroses and abscesses. That is why timely dilution of concentrated drug solutions with water by 2–10 times increases injection safety. It is proposed to include the assessment of osmotic activity and local irritant effect of drug solutions in the standard of drug quality control.
Introduction. One of the most progressive directions of the modern stage of development of biology is the deepening of knowledge about the mechanisms of regulation of metabolic processes, in particular about signal molecules that transmit information to the cell through ion channels and nuclear receptors associated with G-protein or with enzymatic activity. The nuclear Farnesoid X receptor (FXR) is mainly expressed in the liver and intestines, it regulates key genes that provide the processes of synthesis, transport and reabsorption of bile acids, and is also involved in the metabolism of lipids and carbohydrates.
Aim. To evaluate the effect of a farnesoid X receptor agonist on postprandial lipemia in rats fed a supraphysiological fat diet.
Materials and methods. An experimental, prospective, controlled, unblinded, randomized study was conducted to study the effect of a farnesoid X receptor agonist (obeticholic acid) on postprandial lipemia in rats receiving a diet containing a supraphysiological dose of fats.
Results and discussion. It has been shown that when assessing postprandial lipemia, an oral test for tolerance to supraphysiological doses of fat with the determination of the initial lipid profile parameters and 4 hours after exercise has a sufficiently high information content. It was found that in animals that received a diet containing an increased amount of fat for 28 days, there was an imbalance in lipid metabolism with activation of their absorption in the intestine, but a "slow" reaction of the mechanisms of intermediate lipid metabolism, which was accompanied by the accumulation of triglycerides and cholesterol in the blood of hungry rats, chylomicrons and LDL. At 4 hours post-feeding, these animals showed abnormal increases in triglycerides and cholesterol.
Conclusion. The use of obeticholic acid harmonizes lipid metabolism against the background of alimentary fat load, due to the activation of farnesoid X-receptors of the intestine and liver, which is manifested by a simultaneous increase in the intensity of lipid absorption processes and their intermediate metabolism. As a result, the risk of hyperchylomicronemia, hypercholesterolemia and hypertriglyceridemia is eliminated, the likelihood of developing secondary hyperlipedemia, insulin tolerance and functional overload (or pathology) of the liver is reduced.
Introduction. Radioresistance of cancer cells is a serious problem in radiation therapy of tumor diseases. Radiosensitizers make malignant cells more sensitive to radiation and increase the effectiveness of radiation therapy; however, their widespread clinical use is limited by significant side effects. The development and study of new radiosensitizers seems to be an urgent task of modern pharmacology.
Aim. The purpose of this work was to study the effectiveness of lithium ascorbate as a radiosensitizer under the influence of photon and neutron radiation in wide dose range.
Materials and methods. Evaluation of the biological effect was carried out using the tumor line of prostate cancer PC-3. We used a cyclotron to produce neutron radiation and a Cobalt-60 source to produce gamma radiation.
Results and discussion. We have proved an increase in the cytotoxic effect with the combined use of different types of ionizing radiation and lithium ascorbate. The resistance of the prostate cancer line to gamma radiation at an absorbed dose of 0.5–3.0 Gy was revealed. It was shown that tumor cells of prostate cancer are more sensitive to the effects of the study drug in minimal concentrations in combination with neutron irradiation compared to gamma radiation in the same absorbed dose. The main mechanism of the radiosensitizing action of lithium ascorbate is the local induction of oxidative stress, which synergistically enhances the action of ionizing radiation.
Conclusion. The combination of lithium ascorbate with neutron radiation leads to a more pronounced resulting cytotoxic effect. An increase in the concentration of lithium ascorbate led to the pro-oxidative action with an increase in the damaging effect on cells.
Introduction. Trastuzumab is the first known anti-HER2 agent, which revolutionized the treatment of one of the most common cancer types – breast cancer. Despite trastuzumab being approved long time ago, further improvement of related analytical methods remains relevant primarily due to the emergence of new biosimilars. For instance, immunogenicity – adverse reaction which is usually associated with biological drugs, can still be relevant for trastuzumab. Anti-drug antibodies, including neutralizing antibodies, caused by trastuzumab therapy, can affect drug effectiveness and safety profile.
Aim. The aim of this study was to develop and validate the analytical method for neutralizing anti-trastuzumab antibodies determination in human blood serum.
Materials and methods. The neutralizing anti-trastuzumab antibody determination was carried out by the competitive ELISA method, using spectrophotometric detection in the visible range of the spectrum.
Results and discussion. The developed method was validated for cut-point, selectivity, sensitivity, specificity, precision and stability (short-term and long-term). To decrease the background noise from non-specific binding of sera components, the minimum required dilution value was determined at 0.5 % serum. The calculated value for cut-point was 14.62 %. The sensitivity of the developed method was estimated at 1985.2 ng/mL of neutralizing anti-trastuzumab antibodies.
Conclusion. The obtained results allowed us to apply the developed ELISA method for the neutralizing anti-trastuzumab antibodies determination in human blood serum during trastuzumab immunogenicity assessment in bioequivalence clinical trials.
REGULATORY ISSUES
Introduction. Providing high-quality, effective and safe drugs intended for the treatment of rare (orphan) diseases among the population of the Member States of the Union is one of the most significant and promising development vectors for manufacturers-developers. Ensuring the availability of medicines (MP) for patients suffering from rare diseases should be regulated by state incentives for the development and launch of orphan drugs on the market by domestic manufacturers through updating and timely updating of regulatory legal acts in the field of drug registration, as well as providing benefits when initiating the registration process.
Text. The article assessed the possibilities and prospects for the introduction of orphan drugs into circulation within the framework of the Eurasian Economic Union for domestic manufacturers.
Conclusion. A review of the possibilities and prospects for launching orphan drugs for domestic manufacturers indicates the need to develop regulatory and legal regulation of aspects of pharmaceutical development in order to increase the availability of treatment for patients with rare diseases, both at the level of the Russian Federation and within the legal framework of the Eurasian Economic Space.
DISCUSSIONS
Introduction. Computational chemistry methods and, particularly, the noncovalent molecular docking are increasingly implemented into the practice of drug development. Previously, a risk management of potential biases did not applied for this relatively young research instrument.
Aim. The study objective was to design the risk assessment system for noncovalent molecular docking.
Materials and methods. The development of bias risk assessment system was based on the world's leading practices in noncovalent molecular docking.
Results and discussions. As a result of the deductive analysis of the molecular docking process, bias domains were identified and a risk-based algorithm was proposed, which was tested on a sample of articles obtained during a systematic review. A tendency to frequent limited provision of information on the methodology of the computational experiment, as well as on the application of practices proven to lead to irrelevant results of molecular docking, has been revealed.
Conclusion. The data obtained cannot be extrapolated to all studies that refer to the results of molecular modeling. However, through the proposed risk-based algorithm, the attention of researchers is focused on assessing the quality of such publications. We hope that the developed tool for bias risk assessment in noncovalent molecular docking will be finalized and eventually put into practice. It will possibly reduce the share of low-quality work in the field of drug development at the earliest stages.
CONFERENCE PROCEEDINGS
Introduction. This publication represents the materials of the traditional scientific conference of senior students of the Institute of Pharmacy of the Sechenov University and the Faculty of Fundamental Medicine of the Lomonosov Moscow State University under the general motto "Drug. Medicine or... Difficulties in translation", which took place on May 27, 2022. Every year the topic of such a conference is the most pressing medical and social problems related to the effectiveness and safety of medicines. This conference was timed to coincide with the 10th anniversary international congress on diseases of the head and neck organs, which was taking place at that time, and was devoted to the problem of pharmacotherapy of oncological diseases.
In Russia, as well as in the world, oncological diseases are widespread and occupy the second place among the causes of death. So, according to statistics, every 40th person in Russia has an oncological disease.
Thousands of researchers around the world are striving to understand the etiology of cancer, improve the efficiency of diagnosis and treatment of the disease, ensuring slow but steady progress in solving these problems.
Early diagnosis and timely targeted treatment are the key to successful therapy and a favorable outcome.
The presentations discussed the main directions in the development of anticancer drugs that provide greater efficacy and safety of treatment, modern approaches to early cancer diagnosis using microRNA as tumor markers. Of great interest was the report on the possibility of predicting the toxicity of drugs even before the stage of chemical synthesis on the basis of computer-aided drug design. Also, attention was paid to the problems of palliative care and rehabilitation of cancer patients, psychological, ethical and legal aspects of this. Discussion and medical assessment of these issues will certainly arouse the interest of a fairly wide audience. The following are only three reports from those that have sounded, giving an idea of the "classical" methods, a new direction in treatment, and the technological implementation of targeted tumor therapy.
ERRATUMS
Drug development & registration. 2022;11(2):59–64. (In Russ.) https://doi.org/10.33380/2305-2066-2022-11-2-59-64. Published: 25.05.2022.
ISSN 2658-5049 (Online)